Quality management software solutions for the world of medical devices. We are a company that has developed itself from a pioneer in the field of quality-related software back in the 1980s to one of the global technology leaders for management software solutions today.
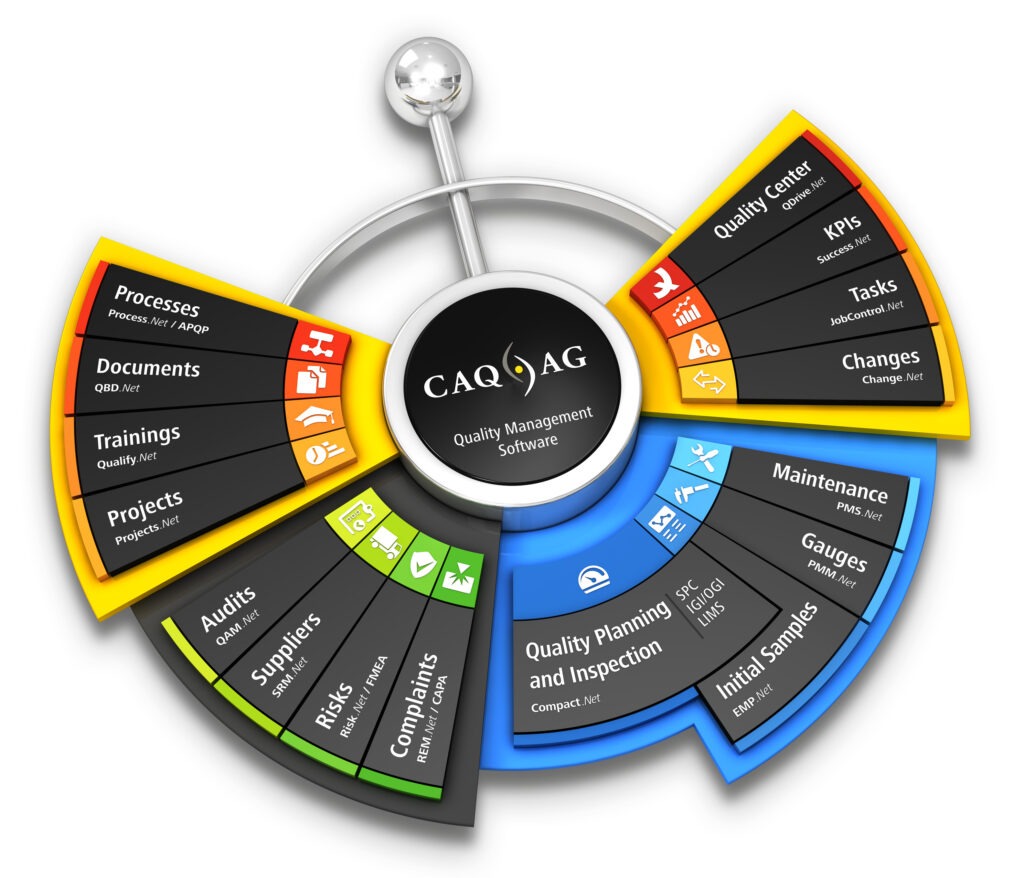
We see ourselves as tool and solution provider for our customers, as our software enables them to implement superior processes and produce high-quality goods. As the product quality has a direct impact on the success of a company, we believe that we and our solutions directly contribute to said success. Hence, we are not only proud of our own products, but primarily proud of the products of our customers. We aim to always provide our customers with the best, most sophisticated and up-to-date software solutions the market has to offer, in order to support them on their journey to success.
Or in other words: What do Germany’s most successful mineral water, most traditional pencil factory, most exported sparkling wine brand, most renowned medical technology companies, and two of the world’s five largest automotive suppliers have in common? They all rely on our CAQ.Net® software solutions.
Decades of Experience
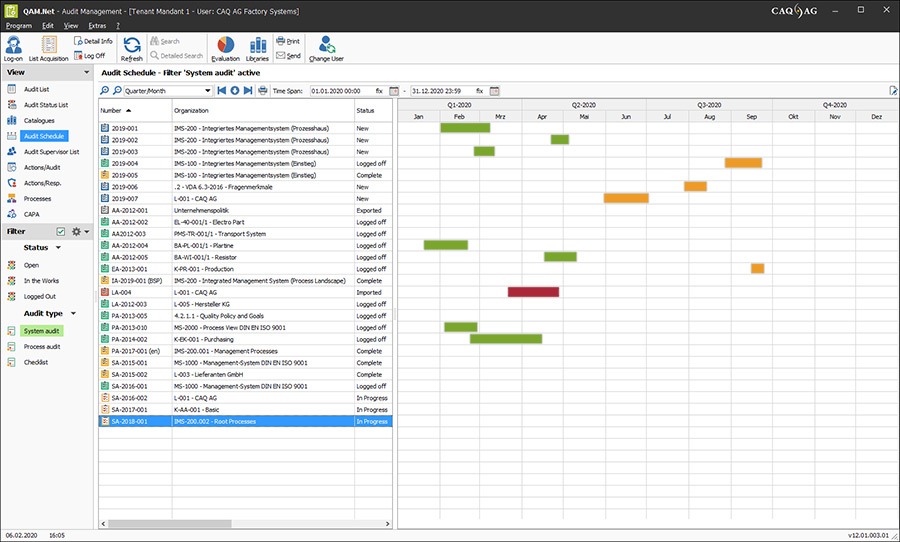
We have over thirty years of experience in the area of computer aided quality assurance and quality management for manufacturers and suppliers of medical technology. Over the decades we have continued to support said customers in the implementation and maintenance of our standard-compliant software solution and many of these customer relationships have since developed into knowledge-sharing partnerships. These partnerships allow us to continuously improve our product and rapidly adapt to changing industry demands.
Target/Actual Comparisons with Market Demands
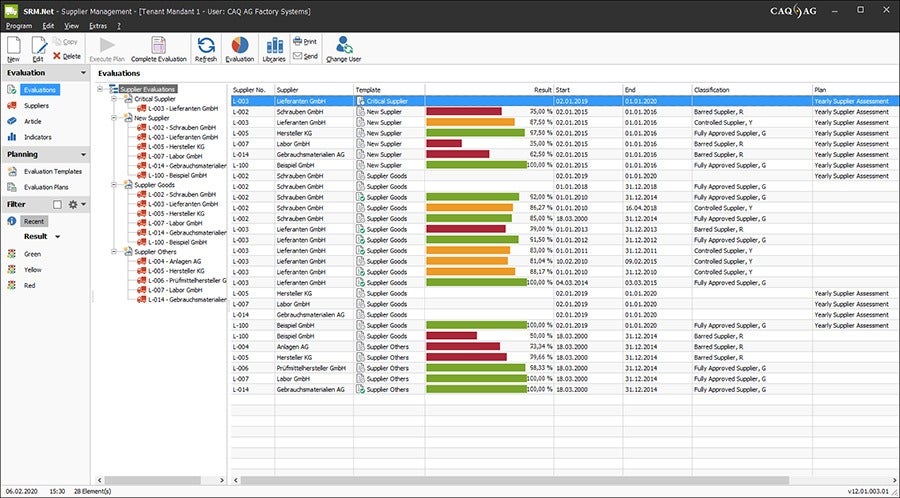
We also work in close cooperation with a variety of partner companies who are specialized in qualification and validation procedures in the medical technology field. The resulting target/actual comparison between our software and the most current requirements by manufacturers and suppliers of medical technology means that our customers are always in command of a cutting-edge software solution that allows professional, efficient, and standard-compliant quality management.
A Holistic Management Solution
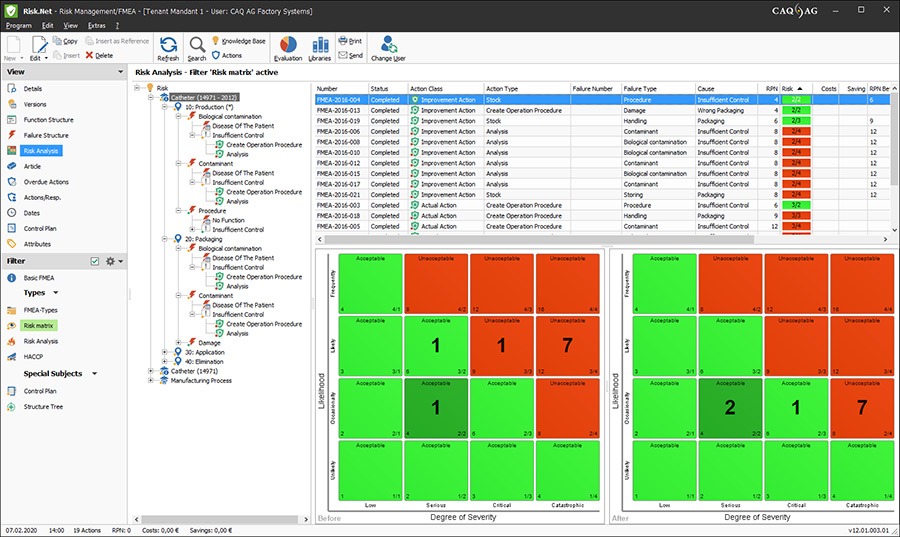
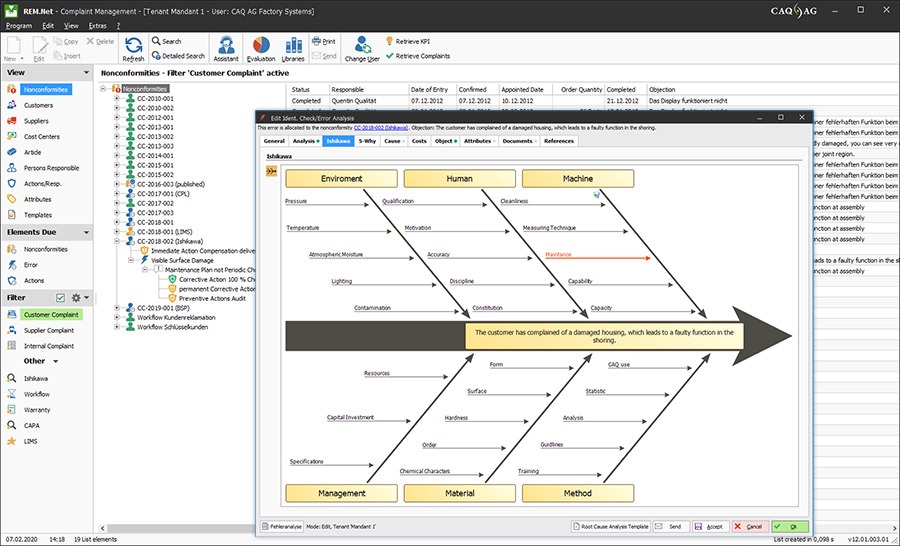
Successful quality assurance and management in the field of medical technology requires an all-inclusive management solution that covers all quality-related facets from product design to complaint management and safeguards consistent compliance and traceability. This solution must also incorporate all aspects of CAPA management and facilitate the adherence to valid standards and guidelines. This solution is our software CAQ.Net®: your all-inclusive management solution for the medical technology sector.
Or to put it short: We are your reliable partner in all matters relating to quality and are here to support you throughout whatever the digital future may bring.
CAQ AG Factory Systems
In der Wester 5
55494 Rheinböllen, GERMANY
Phone: +49 6764 90200-0
E-Mail: Info@CAQ.de
Web: www.CAQ.de/en