We all conduct risk analysis in our everyday lives. Every time we weigh up the probability of something happening and the resulting degree of severity, we are conducting risk analysis. If you leave the house when it is raining, the prospect of getting wet is very high. The degree of severity, however, is low. You’ll simply get wet. If you don’t put on your safety belt in the car, the probability of something happening is comparatively low. However, if something does happen, the severity of what may happen can be dramatic.
Within the area of risk management, techniques such as FMEA, 3D signal light factors, HACCP, or ISO 14971 risk matrices are used in order to weigh up precisely these types of correlation between probability and severity, and to ascertain appropriate risk parameters. Whether automotive, medical technology, or food industry: in Risk.Net you have the best methods of risk analysis of all sectors at your disposal, so that you can identify, analyse and classify all risks and dangers.
It provides you with everything you require for conducting effective risk analyses. Use the comprehensive functions of the software in order to identify, evaluate, and document relevant risks and thereby fulfil key requirements of customers and regulating authorities.
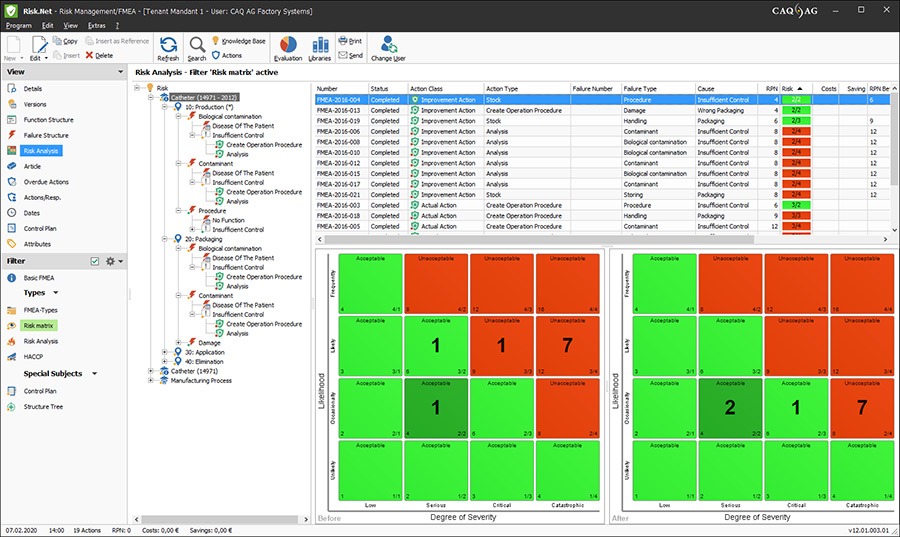
The before/after risk matrix in Risk.Net, for instance, allows you to conduct effective and precise risk management in accordance with ISO 14971. It allows you to schematically track the effectiveness of inspections or alterations of construction that have been carried out. By comparing before and after, you can easily track the effectiveness of the measures you have taken and prove their effectiveness at any time, because if the frequency of an error in a product decreases after a change has been made, this will show up in the ‘after’ matrix.
More information on our website: https://www.caq.de/en/Software/FMEA_Risk_Management