Medovate's managing director believes SAFIRA can make regional anaesthesia safer and more cost-effective by turning it into a one-person procedure
Around 20 million regional blocks are carried out in Europe and the US every year (Credit: Wikimedia Commons)
UK medtech company Medovate is hoping to bring a device to market in 2020 that will revolutionise regional anaesthesia, according to its managing director.
By giving an anaesthetist full control when injecting a local anaesthetic, it aims to reduce the number of mistakes commonly made by assistants, making the practice safer and more cost-effective.
SAFIRA (safer injection for regional anaesthesia) is a small device that attaches to a syringe and connects it to an operating system — this could be a foot pedal, hand operator, or an integrated needle controlled using buttons.
This allows the anaesthetist to single-handedly carry out a regional block procedure, in which an injection is used to numb a specific area, for example, the leg during knee replacement surgery.
By removing the need for an assistant to administer the anaesthetising drug, this reduces the number of mistakes made during regional block procedures, making it safer, and more cost-effective because fewer follow-up appointments are needed.
Medovate hopes SAFIRA will reach the market and be approved for use in both Europe and the US in early 2020 — less than two years after the company’s inception in 2018.
Managing director Stuart Thomson claimed that for a company to have its first product launch from scratch in such a short space of time is virtually unheard of in the medtech industry.
He said: “We’re proving that innovation is alive and well in the National Health Service by building an exciting pipeline of technologies in anaesthesia, surgery, intensive care and airways.”
The regional anaesthesia device is one of several innovations Medovate is working on
Thomson was previously involved in the NHS innovation pipeline and worked on getting new healthcare technologies to market in the UK.
But he claimed a lack of funding made this process too slow and inefficient, so he decided to create somewhere several medical innovations could be housed and developed in the same place.
After receiving £9m ($11.5m) of funding from UK investor NVM Private Equity and a private US investor, he launched Medovate in February 2018.
As a medtech development company, Medovate works with NHS partners to take medical devices in the categories of anaesthesia, surgery, intensive care and airways from development to clinical trials and ultimately regulatory approval.
It does this by taking ideas and products created within the NHS and offering hospitals the financial, technical and management support necessary for these medical devices to reach the commercial market.

If SAFIRA gains regulatory approval, it will be the first medical device the company has brought to market since its inception — although Thomson hopes innovations in other areas will soon follow.
As well as making the regional anaesthesia device widely available in 2020, Medovate will attempt to bring Humidicare — a heat and moisture exchanger (HME) that reduces the risk of a patient’s airways becoming blocked while on a ventilation system — to market shortly after.
The company has taken on six technologies from different NHS hospitals since February 2018, and is hoping to introduce two more devices in 2021 following clinical trials.
Why SAFIRA is needed in regional block procedures
A regional block procedure involves anaesthetising a specific area of the body by blocking nerve sensations, meaning patients can remain pain-free during invasive surgery without requiring a general anaesthetic.
Examples of these procedures include numbing the leg for a knee replacement operation, or the arm before hand or wrist surgery.
The process of administering a regional block normally requires two people — an anaesthetist to insert a needle into the patient’s body, and an assistant to operate the syringe containing a local anaesthetic.
After inserting the needle, but before administering the drug, the anaesthetist will ask their assistant to aspirate with the syringe to ensure they haven’t entered a blood vessel.
If no blood enters the syringe, the assistant is asked to inject 1ml of anaesthetic to see if there is any “push back”.
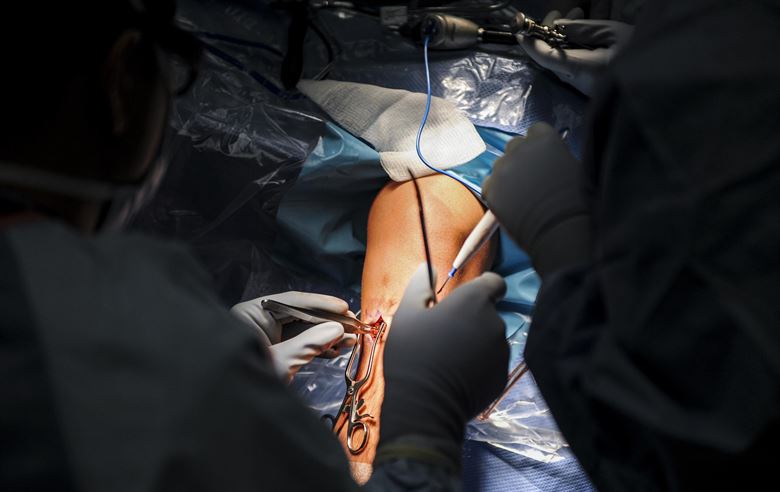
Push back on the syringe’s plunger indicates pressure, meaning the needle is either in, or too close to, a nerve fascicle — a bundle of nerves in the peripheral nervous system — rather than an area of soft tissue where the anaesthetic should be injected.
Injecting anaesthetic solution into an area where the pressure is above 20 PSI can cause serious, long-lasting nerve damage where the patient loses use of the limb.
As well as the significant impact on the patient’s life, it costs the NHS in the UK around £30,000 per case — in the US, this is closer to $500,000.
Thomson said this occurs roughly once in every 1,000 regional block procedures, although transient nerve damage where the patient feels tingling in the area following the operation is more common, happening between 4% and 5% of the time.
While transient nerve damage is less severe, it still costs the healthcare system money, requiring follow-up appointments and potentially further tests.
How does Medovate’s device make regional block procedures safer and more cost-effective?
At the moment, there is no way of telling for certain who is at fault for causing the nerve damage — SAFIRA removes this ambiguity by making the regional block a one-person procedure.
Because the device allows the anaesthetist to perform a regional block single-handedly — including aspirating beforehand — any mistakes made can only be attributed to them, rather than the assistant or surgeon who operates.
Thomson believes that from both a medical and legal angle, SAFIRA allows the anaesthetist to say with confidence that they performed the procedure safely, adding that it could be “revolutionary” for the practice of regional anaesthesia.

Giving the anaesthetist total control and streamlining the procedure makes it much cheaper by also reducing theatre time — Thomson claims analysis from a University of East Anglia health economist found that SAFIRA could save up to £80 ($102) per regional block.
SAFIRA also makes the procedure safer by automatically shutting down and preventing any injection if it detects a PSI reading above 17 — three units below the maximum pressure level at which anaesthetic can be safely administered.
