Capable of delivering results in minutes, the MicroGEM Sal6830 SARS-CoV-2 Saliva Test is said to be the country’s first point-of-care PCR saliva test for COVID-19
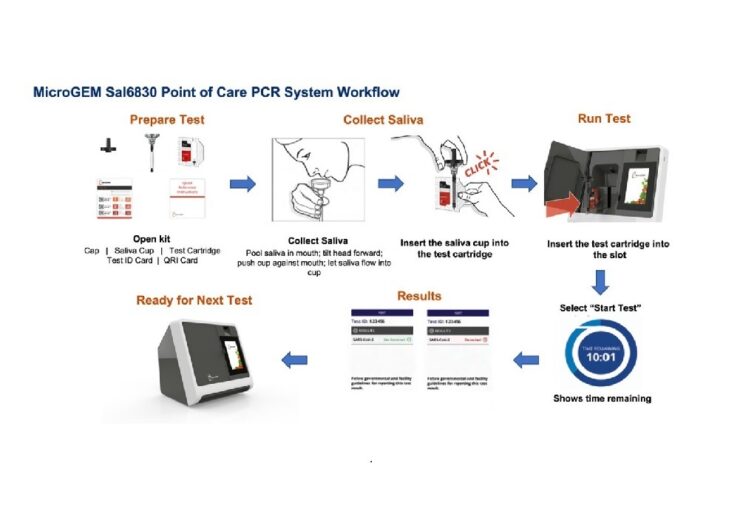
MicroGEM Sal6830 SARS-CoV-2 Saliva Test procedure explained. (Credit: MicroGEM International PLC)
Molecular biology company MicroGEM US has received emergency use authorization (EUA) from the Food and Drug Administration (FDA) for its MicroGEM Sal6830 SARS-CoV-2 saliva test.
With the EUA, the MicroGEM Sal6830 SARS-CoV-2 saliva test is the country’s first point-of-care polymerase chain reaction (PCR) saliva test delivering results in 27 minutes.
The test, which was clinically tested during the Delta and Omicron waves of the pandemic, has the ability to detect current variants. It is also able to protect against obsolescence from future variants.
According to the firm, the MicroGEM test is easy to operate and has a simple saliva collection technique to reduce the exposure of healthcare workers to the samples.
The test is designed to deliver results with just a small amount of saliva, making it more comfortable for patients in comparison to the traditional swab testing method.
Its small size facilitates easy installation at testing sites such as mobile testing labs, ambulatory surgical centres, emergency departments, and CLIA-waived testing sites at the workplace.
With a cartridge design, the MicroGEM Sal6830 Point of Care PCR System allows new targets to be rapidly inserted or swapped, considerably lowering both assay and product development time.
MicroGEM CEO Jeff Chapman said: “The MicroGEM Sal6830 SARS-CoV-2 Saliva Test will be an essential testing tool in our ongoing efforts to get our nation’s communities and businesses back to regular operations.
“The introduction of the MicroGEM Sal6830 Point of Care PCR System marks a historic step in our mission to democratize molecular diagnostics by moving ultra-fast, high-performance testing out of laboratories and closer to people at the point of need, thus allowing decisions to be made in real time.”
The FDA has granted EUA to the MicroGEM’s test only for the detection of nucleic acid from SARS-CoV-2 by authorised laboratories only.
The development and launch of the PCR System and saliva test have been funded in part by the National Institutes of Health (NIH), Rapid Acceleration of Diagnostics (RADx) initiative.
