The US Food and Drug Administration (FDA) has approved Masimo’s RAS-45, an acoustic respiration sensor for rainbow Acoustic Monitoring (RAM), for infant and neonatal patients.
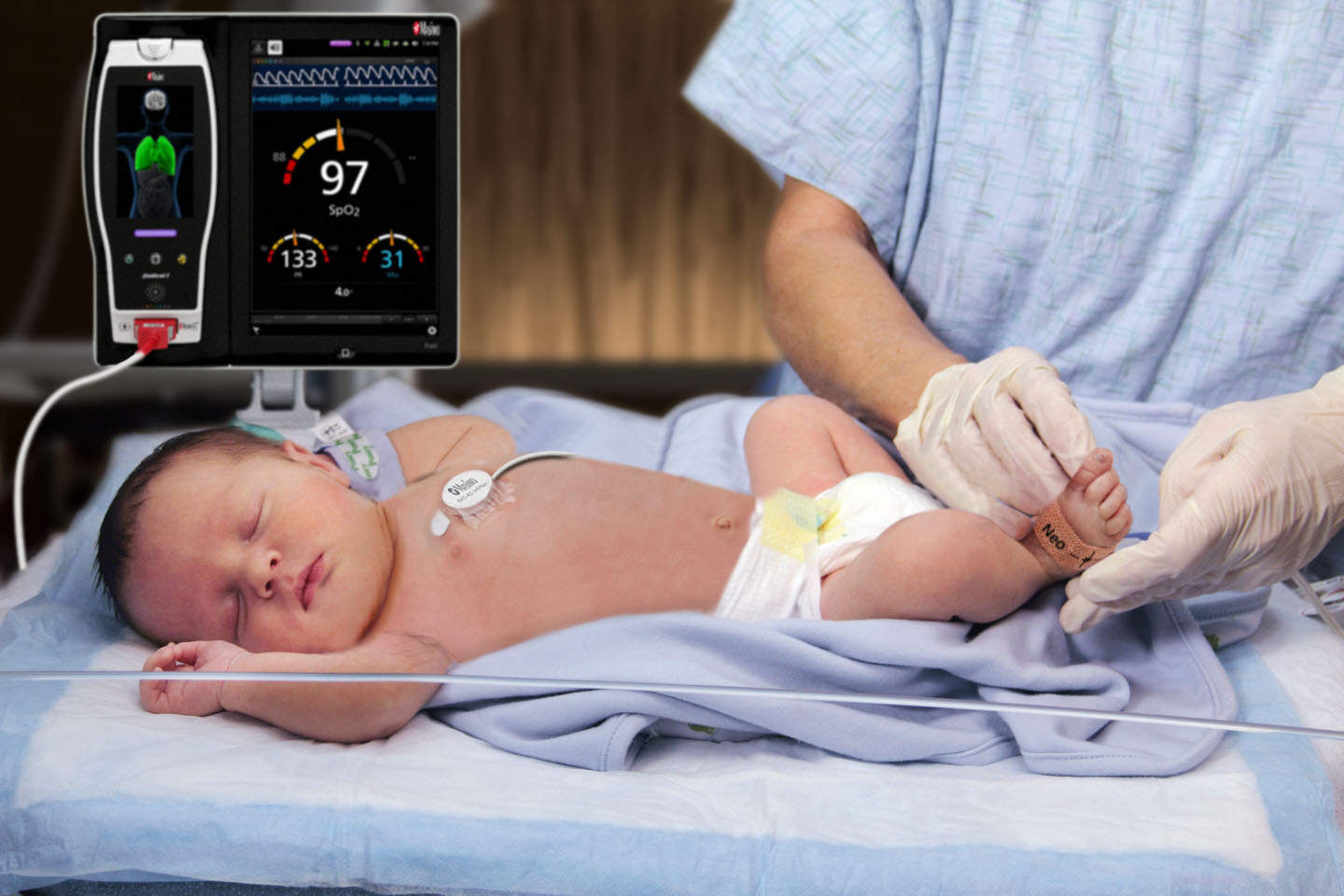
Image: Masimo Root with Radical-7, RRa, and the RAS-45 Infant/Neonatal sensor. Photo: courtesy of Business Wire.
Earlier, only patients over 10 kilograms were eligible for RAM sensors, but wit the new clearance acoustic respiration rate measurement is now possible for patients of all sizes, including neonates, in the US.
This is partially due to the sensor’s maximum breathing rate being expanded to 120 breaths per minute while maintaining accuracy within +/- 1 breath per minute.
RAM, available on most rainbow SET-ready platforms, measures respiration rate noninvasively using an adhesive sensor with an integrated acoustic transducer, the RAS-45 and RAS-125c, applied to the patient’s neck area or, for infant and neonatal patients under 10 kg, the chest.
The respiratory signal is separated and processed by leveraging acoustic signal processing that uses Masimo Signal Extraction Technology (SET) to display continuous respiration rate (RRa) and an acoustic respiration waveform.
Clinicians can listen to the sound of a patient’s breathing by using the acoustic sensor, whether at the bedside, via a point-of-care device like the Radical-7 Pulse CO-Oximeter, or remotely, from a Patient SafetyNet view station.
The sensor is smaller than the RAS-125c sensor, and with a diameter of about 2.2 cm without adhesive, it is slightly larger than a nickel.
Masimo founder and CEO Joe Kiani said: “From the beginning, we have focused our R&D on neonates and children for many reasons, including our belief that helping clinicians care for children will provide more benefit to society.
“RAM harnesses the power of our breakthrough signal processing and sensor technology and applies it to a measurement that has either been unreliable or difficult to use, respiration measurement, the third vital sign.”
Earlier this year, Masimo secured FDA clearance of Next Generation SedLine brain function monitoring.
With SedLine, clinicians can monitor the state of the brain under anesthesia with bilateral acquisition and processing of four leads of electroencephalogram signals.
The next generation SedLine features an improved signal processing engine, driving several performance enahncements, giving clinicians a more complete picture of the brain.
