All articles by Surya Rao Akella
Cerus files for CE Mark registration for INTERCEPT RBC system
Cerus president and CEO William ‘Obi’ Greenman said “The INTERCEPT RBC CE Mark submission is a major milestone in the…
Biomax Informatics introduces NeuroXM Brain Science Suite
Biomax Informatics AG announced the launch of its latest product, the NeuroXM Brain Science Suite, this week at the Biomax-ETRI…
Earth Science Tech unveils positive results from Hygee medical device
Earth Science Tech said that its Hygee is a wearable medical device that allows women to anonymously test for sexually…
Avinger announces milestone in patient treatment with Pantheris device
The next-generation Pantheris was launched on a limited basis in the US following receipt of 510(k) clearance from the US…
Endonovo announces further advancement of SofPulse product
Endonovo Therapeutics CEO Alan Collier said: “We are pleased to announce the positive performance of our SofPulse units which have…
Cytel launches East 6.5 industry standard platform for clinical trial design
Cytel said since 1995, East has empowered trial sponsors of all sizes to optimize study planning and monitoring efforts, accelerate…
Francis Medical completes $18m round of Series A financing
Arboretum Ventures led the Series A with co-investments from H2Oey Ventures, an affiliate of Solas BioVentures Fund, Tonkawa and Boston…
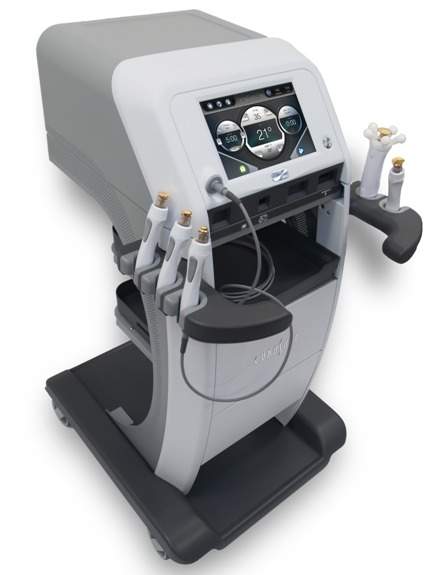
Hologic introduces TempSure Surgical RF technology in North America
According to Hologic, the new TempSure RF platform enhances the ability of clinicians in performing both surgical and non-surgical aesthetic…
Check-Cap wins FDA conditional approval to begin pilot study of C-Scan in US
FDA’s conditional approval for the IDE requires Check-Cap to provide additional information to the FDA and the company can start…
UK’s NICE recommends non-invasive MRI scan for prostate cancer
NICE stated that mpMRI creates detailed images of the prostate gland to allowclinicians to better identify suspected prostate cancer. Presently,…