
From digital stethoscopes to an AI-driven virus tracking system, a raft of new innovations have come to the fore as scientists take the fight to Covid-19.
Modern technologies available to researchers have helped them advance the global effort to not only find a vaccine, but to track and trace infected patients, diagnose those who have the disease and treat the worst symptoms.
As the world tries to tame a pandemic that has changed life as we know it for many and dismantled economies, we take a look at seven of these innovations that have played a role so far.
Technological innovations to come forward during Covid-19
BillionToOne’s DNA molecule-matching technology
BillionToOne, founded by Princeton University graduates Dr Oguzhan Atay and David Tsao in 2016, is a diagnostics company focused on genetic testing.
The start-up says its technology can measure DNA in 1,000 times more detail, creating an accurate diagnostics tool that can track changes inside the body at a molecular level.
On 7 April, the firm unveiled its Covid-19 test protocol, which it claims will enable one million tests for the virus to take place a day in the US alone.
Speaking at the time, BillionToOne’s Dr Atay said: “We’re honoured to be on the front lines of this fight against the pandemic, and we’re certain this unique technology will help save lives and stop the spread of the virus.”
Using its qSanger spike-in technology — which is designed to bind a DNA molecule with a matching sequence — followed by data analysis to test whether the virus is present, it can automatically perform 3,840 assessments a day.
Its test uses different sets of instruments and chemicals from existing Covid-19 diagnostics, enabling labs to increase their testing capacity, as well as engaging sanger sequencers.

Most of the molecular level testing during the pandemic is being conducted through the polymerase chain reaction (PCR) method, which is used to directly detect the presence of an antigen, rather than the presence of the body’s immune response.
However, Dara Lo, a medical devices analyst at analytics firm GlobalData, offers a word of caution, saying: “The novel protocol that BillionToOne’s Sanger sequencing Covid-19 test uses also puts it at risk for errors in accuracy, meaning it could produce less accurate results compared with the current Covid-19 PCR tests
He added: “Despite the estimated availability of 700 to 800 sanger sequencers in the US — which would allow for BillionToOne to unlock over one million Covid-19 tests a day — labs carrying out Covid-19 tests need to be certified for the highest level of safety and containment.
“Sanger sequencers are currently used for genome sequencing, and not certified to be handling live viruses, which means that these sequencing facilities would need engineering changes to meet safety standards.”
Northeastern University uses nanoparticles to try beat Covid-19
In 2014, the Ebola virus made headlines across the globe as it swept through West Africa, killing more than 10,000 people.
One person who was searching for a technology that could fight the disease was nanomedicine expert Thomas Webster.
A professor of chemical engineering at US-based Northeastern University, his team developed nanoparticles that could be attached chemically to the virus, stopping it from spreading.
Six years and another viral disease later, Prof Webster is once again turning to the nanoscale to fight Covid-19.
He is part of a contingency of scientists contributing ideas and technology to the US’ Centers for Disease and Control Prevention, and is offering nanoparticles as a potential solution to the problem.

Prof Webster is proposing his nanoparticles could attach to the virus and disrupt its structure.
This structural change would halt the virus’ ability to survive and reproduce in the human body.
GlobalData’s director of thematic analysis Kathryn Whitney believes nanoparticles could be a more effective approach to tackling Covid-19.
She said: “Existing antiviral treatments are only partially effective as they attack viruses when they have already infected cells.
“While still in the early stages of development, nanoparticles have the potential to neutralise viruses and prevent infection, which would be a more desirable and effective approach against Covid-19.”
EkoHealth’s Al-driven Covid-19 screening tools through digital stethoscopes
To aid the health service’s response to the pandemic, EkoHealth is using its telehealth technology and infection control tools to allow healthcare professionals to deliver cardiac and pulmonary care to patients.
The California-based start-up — founded by Jason Bellet, Tyler Crouch and Connor Landgraf in 2013 — was set up to enable clinicians to provide the best cardiac care possible.
To do this, the firm manufactures stethoscopes, provider-to-patient software, and AI-powered analysis.
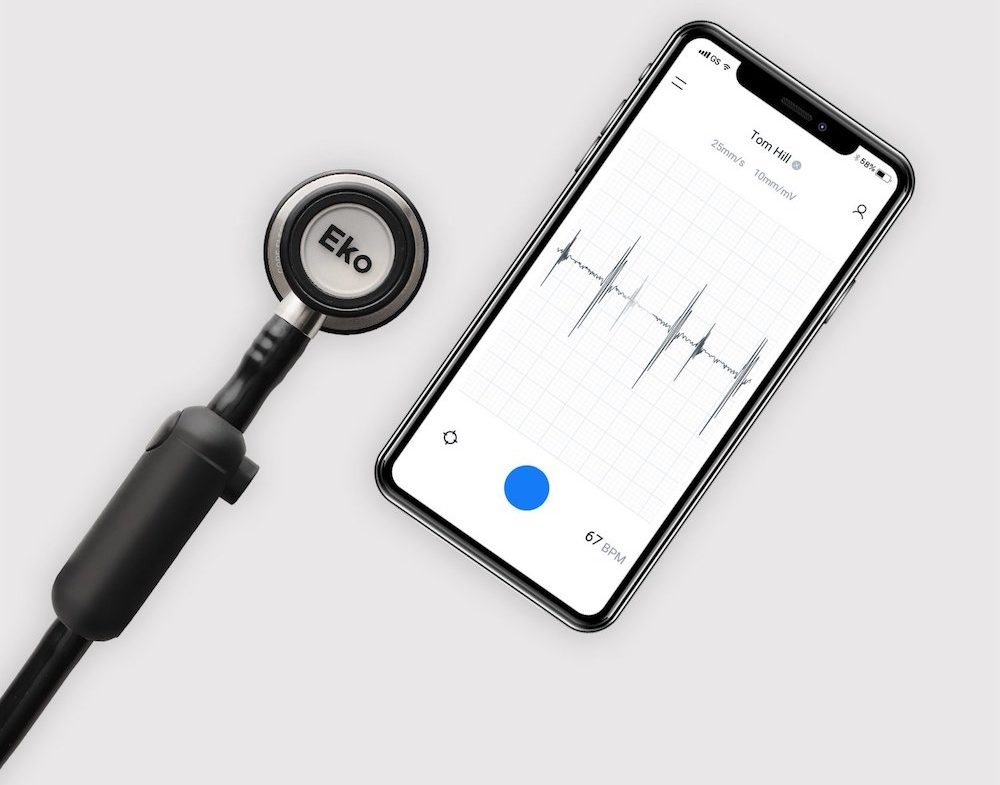
During Covid-19, the company is providing its AI technology and cardiac screening tools to clinicians, offering them real-time visualisations and the ability to analyse and share heart and lung sound data.
The firm’s digital stethoscopes are also aiding healthcare professionals to treat people in the hospital, allowing doctors to detect issues such as abnormal heart rhythms.
MIT’s at-home diagnostics kit
With social distancing a “new normal”, many patients who think they may have Covid-19 are advised to stay away from hospitals unless their condition worsens — making it difficult for healthcare professionals to get a clear picture of a person’s state of health.
To ease the burden on healthcare facilities in the US, university MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) has developed remote patient monitoring (RPM) technology that allows doctors to remotely monitor Covid-19 patients.
The device is a WiFi-like box that analyses wireless signals in the environment, using AI to view people’s vital signs, sleep and movement.
Called Emerald, it can non-invasively monitor patients, which in turn enables a doctor to remotely track their progress by looking at metrics such as breathing and walking speed.
It can also help detect other respiratory problems, for example, whether or not someone suffers from anxiety or has insomnia.
GlobalData medical devices analyst Aliyah Farouk believes this type of RPM equipment will be adopted worldwide.
She said: “This will especially be the case in the US and the UK, as the paradigm of medical practice shifts towards telemedicine.
“RPM shows tremendous promise in improving Covid-19 management and keeping patients out of the hospital.
“The global large-scale implementation of RPM has been limited as most countries lack the regulatory frameworks to authorise and reimburse telemedicine services in emergency and outbreak situations.
“However, the US Food and Drug Administration (FDA) has recently published guidance allowing companies to expand the distribution of hospital devices for use in patients’ homes.
“Numerous companies have been taking advantage of this by producing and repurposing devices to increase their remote functionality.”
BlueDot’s AI-driven virus tracking technology
Digital technology firm BlueDot was founded in 2013 with one purpose — to develop an automated global infectious disease risk assessment system.
The firm was founded by Dr Sharma Khan, who, motivated by his experience as a frontline healthcare worker during the 2003 Toronto SARS outbreak, has been studying the outbreak of infectious diseases over the past 15 years, laying down the scientific foundation for the company’s technology.
Towards the end of 2019, the Toronto-based start-up alerted private sector and government clients about “unusual pneumonia” cases occurring around a market in Wuhan, China.
This was one of the first citings of the virus that became known as Covid-19 — and nine days before the World Health Organization (WHO) released a statement alerting people to the emergence of the novel coronavirus.
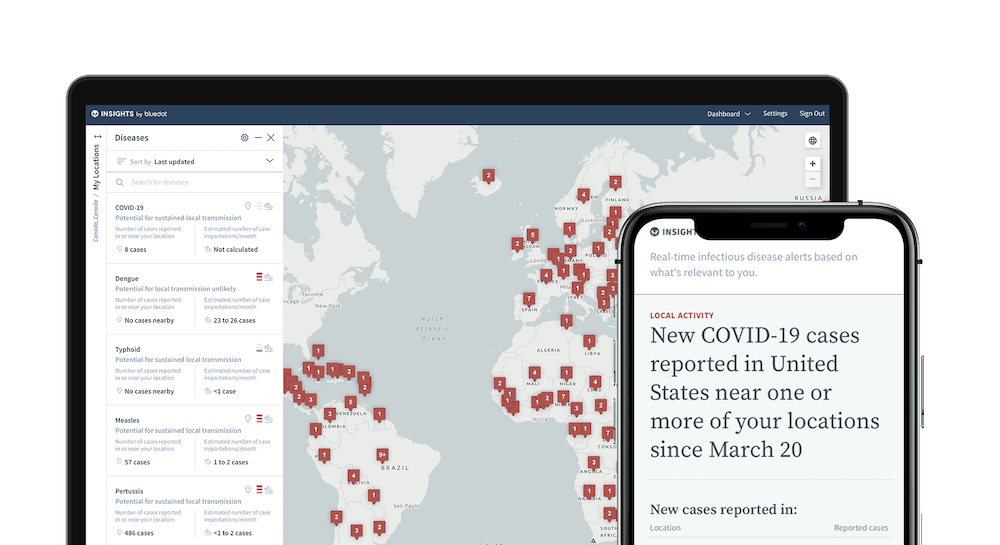
BlueDot’s technology is a software-as-a-service designed to locate, track and predict infectious disease spread.
Its engine gathers data on more than 150 diseases and syndromes around the world, searching every 15 minutes and includes official data from organisations such as the WHO.
Most of the predictive ability is based on data it collects from outside official healthcare sources, such as the worldwide annual movements of more than four billion travellers on commercial flights, alongside human, animal and insect population data.
Combining this data with AI, BlueDot successfully predicted several cities where Covid-19 spread to after it surfaced in Wuhan.
The company is now working with governments and public health agencies from around the world with their Covid-19 responses.
Mesa Biotech’s polymerase chain reaction technology
During this pandemic, the WHO has emphasised the importance of testing populations, which is seen as key to slowing the spread of the virus.
One company involved in increasing testing capacity is Mesa Biotech — a manufacturer of generation molecular diagnostic tests.
Founded by Dr Hong Cai and Dr Bruce Cary in 2009, its technology enables healthcare professionals to access laboratory-quality results at the point-of-care.
During its existence, the San Diego-based company has focused on technology well-suited to dealing with infectious diseases such as Covid-19.
In response to the pandemic, the firm has developed technology that gives test results within 30 minutes.
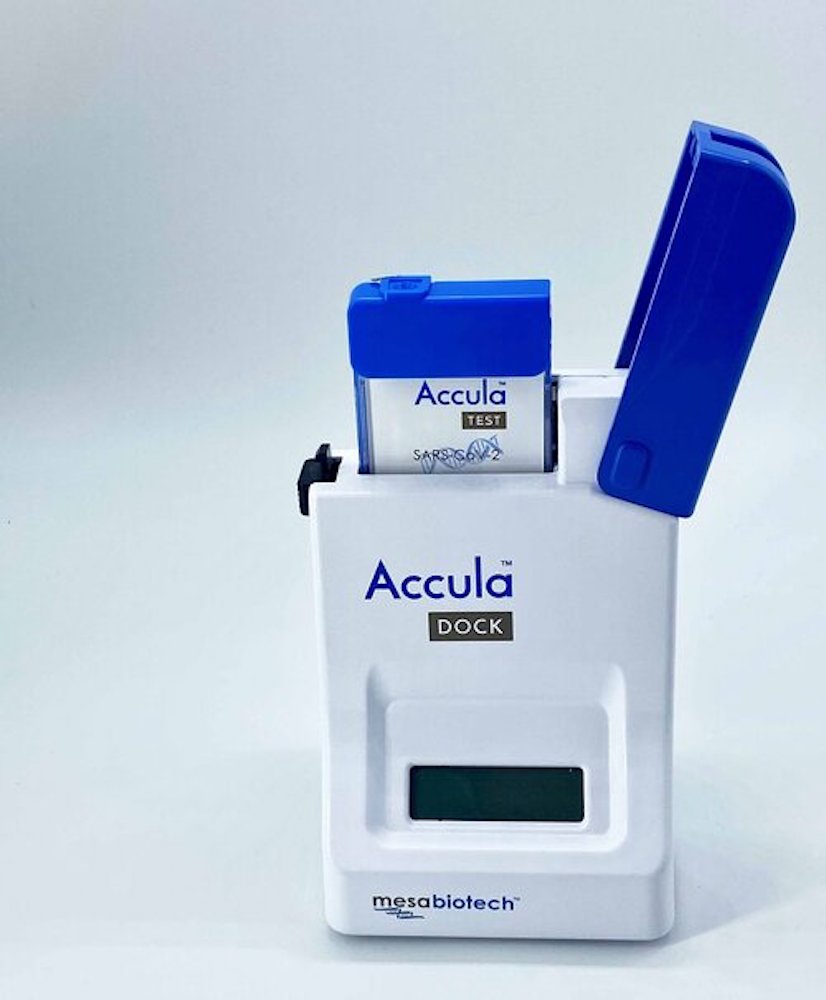
The Accula system can fit in the palm of someone’s hand, allowing many units to be run in the same space, further increasing the speed of Covid-19 diagnosis.
Towards the end of March, the firm received emergency use authorisation from the FDA, allowing the company to begin mass-producing the technology.
At the beginning of April, the company announced it was shipping 10,000 of its Accula tests to healthcare facilities in the US.
SeaStar testing a selective cytopheretic device to treat patients with severe breathing problems
Founded in 2018, SeaStar Medical is a medical device company focused on developing technologies to address life-threatening medical conditions, mainly looking at the excessive inflammation of vital organs.
The firm does this through manufacturing products designed to prevent organ failure that target proteins in the blood that are capable of promoting inflammation.
Inflammation is a key part of conditions such as acute respiratory distress syndrome (ARDS), occurring when lungs become severely inflamed from an injury or infections such as Covid-19.
To help ARDS sufferers with Covid-19, SeaStar Medical is testing a selective cytopheretic device (SCD), which selectively targets proinflammatory blood cells to remove the cytokine storm that causes inflammation.
The company is also investigating how the device could be used to treat people with kidney failure — which an estimated 40% to 60% of Covid-19 patients in intensive care units suffer from.
To carry out the tests, SeaStar received an investigational device exemption supplement approval from the FDA to proceed with a pilot study, which is examining the safety and efficacy of mitigating kidney injury.
Azadeh Laffafian, a medical device analyst at GlobalData, says many hospitalised Covid-19 patients experience acute kidney injury.
She added: “This has led to a surge in demand for dialysis equipment. Continuous Renal Replacement Therapies (CRRT) machines are especially needed and at risk for shortages.
“Other dialysis supplies, in addition to dialysis support staff, are also in high demand.
“According to SeaStar, this device may help reverse and repair kidney injury, thereby reducing the patient’s need for CRRT.”






