The expanded clearance now allows both Momentum and Momentum MIS systems to be used alongside G21 V-STEADY Radiopaque Bone Cement to assist in the restoration of spinal column integrity for patients with advanced-stage tumours
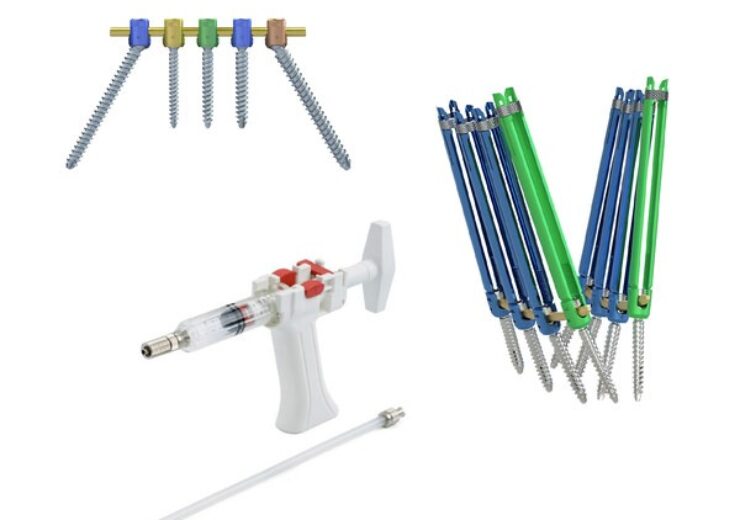
Momentum and Momentum MIS posterior spinal fixation systems and V-STEADY Bone Cement. (Credit: PRNewswire/ulrich medical USA)
ulrich medical USA, a subsidiary of German medical technology company ulrich medical, said that the US Food and Drug Administration (FDA) has expanded the clearance for the Momentum and Momentum MIS posterior spinal fixation systems.
The extended approval permits the utilisation of both Momentum and Momentum MIS systems in combination with G21 V-STEADY Radiopaque Bone Cement to aid in restoring the integrity of the spinal column in patients with advanced-stage tumours.
UCSF Health orthopaedic spine surgery assistant professor Alekos Theologis said: “I’m excited that I can now use Momentum in conjunction with V-STEADY.
“Momentum has been a valuable treatment option and this enhancement will be beneficial to a segment of my patients.”
The Momentum and Momentum MIS posterior spinal fixation systems are employed for stabilising, fixing, and correcting the thoracolumbar and sacroiliac spine.
These systems empower surgeons to address a broad spectrum of adult degenerative and deformity cases, including connections from the occiput to the pelvis, bone cement augmentation, and revisions for adjacent segment disease. They facilitate the restoration of spinal alignment through both open and percutaneous procedures.
ulrich medical USA technology vice president Eric Lucas said: “We are pleased to have received this expanded indication from the FDA.
“The combination of Momentum with V-STEADY offers our surgeon customers a new treatment option to support their most challenging patients.”
In October 2023, ulrich medical USA announced the full commercial launch of the Momentum MIS posterior spinal fixation system in the US. The system, originally introduced at the beginning of the year, had successfully concluded its limited launch phase, said the company.
According to ulrich medical USA, Momentum MIS presents the company with a percutaneous solution tailored for the expanding minimally invasive surgery market.
Designed with a comprehensive tab-based system that includes a narrow profile, extended screw tabs, removable rings for reinforced support, and integrated reduction, the system also introduces an innovative guide-wire lock.
Additionally, Momentum MIS shares the advantages of the Momentum posterior fixation platform, including conical screws with cannulated, fenestrated screw options.
Commercially launched in the US in 2020, the Momentum system comprises polyaxial screws in cannulated/fenestrated and non-cannulated options, along with reduction screws and iliac screws available in open and closed designs.
Additionally, the system includes rods, connectors, and crosslinks. These implant components are versatile and can be assembled in various configurations, allowing customisation for each individual case.
