t:slim X2 insulin pump is the first FDA approved system to provide automatic correction boluses, apart from facilitating adjustment of insulin to prevent fluctuations in blood sugar
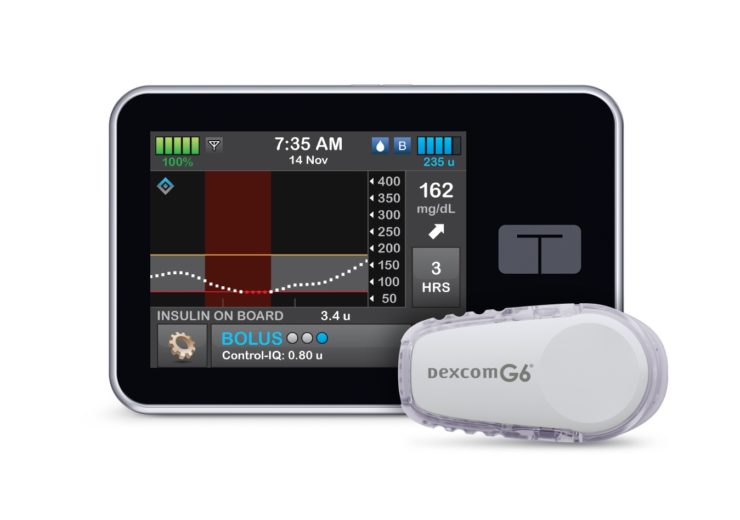
Image: The t:slim X2 insulin pump with Control-IQ technology from Tandem Diabetes Care. Photo: Courtesy of Business Wire.
US-based medical device manufacturer Tandem Diabetes Care, has secured the US Food and Drug Administration (FDA) approval for its t:slim X2 insulin pump, with advanced hybrid-closed loop technology Control-IQ, for increased time in range (70-180 mg/dL).
The t:slim X2 insulin pump with interoperable technology is an alternate controller enabled (ACE) pump designed to deliver insulin at set and variable rates, for diabetes mellitus patients who need insulin.
Tandem Diabetes Care president and CEO John Sheridan said: “With this clearance, we will be launching the most advanced automated insulin dosing system commercially available in the world today. This is a testament to our commitment to improving the lives of people with diabetes by offering simple-to-use products that deliver superior performance.”
T:slim X2 insulin pump is the first FDA approved system for automatic correction boluses
The company said that its t:slim X2 insulin pump with Control-IQ technology is the first FDA approved system to provide automatic correction boluses, apart from facilitating adjustment of insulin to prevent fluctuations in blood sugar.
In addition, the Control-IQ technology is said to be the first automated insulin dosing software in a new interoperable automated glycemic controller category.
The technology automatically adjusts insulin delivery with diabetes, through its connection with an alternate controller-enabled ACE pump and integrated continuous glucose monitor (iCGM).
The system integrates with Dexcom G6 continuous glucose monitoring (CGM), eliminating the need for fingersticks for calibration for diabetes treatment decisions.
The t:slim X2 insulin pump with Control-IQ technology works using CGM values, along with other variables, including insulin on board, to predict glucose levels 30 minutes ahead and adjusts the insulin delivery consequently.
According to the company, Control-IQ technology offers optional settings for sleep and exercise that facilitate changes in the treatment values to suit the varied physiologic needs during the activities.
Dexcom president and CEO Kevin Sayer said: “The approval of the Control-IQ system with Dexcom G6 CGM brings together two incredible products to deliver a powerful automated insulin dosing solution for people with diabetes.
“Sensor accuracy is a critical component for automated insulin dosing, and we are excited that Dexcom G6 users can benefit from our collaborative effort to integrate this advanced hybrid closed loop system.”
