The REGENETEN implant is delivered arthroscopically through a small incision over the location of the rotator cuff tendon injury
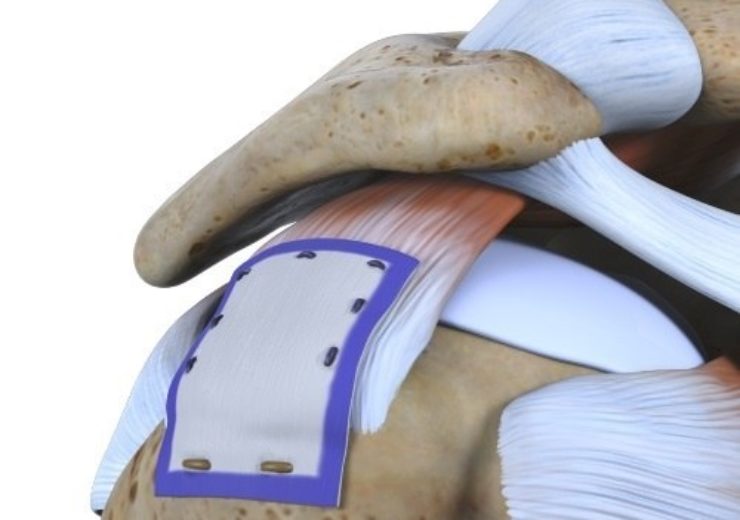
Image: Smith+Nephew's REGENETEN Bioinductive Implant. Photo: Courtesy of Smith+Nephew.
UK-based medical equipment company Smith+Nephew announced that a new clinical study demonstrated positive results for its REGENETEN Bioinductive Implant in treating large (3-5cm) and massive (more than 5cm) rotator cuff tears.
The medical devices manufacturer said that the non-comparative clinical study of the REGENETEN implant in 23 patients with large and massive tears in the US has showed a 96% tendon healing rate in two years.
Smith+Nephew R&D president Vasant Padmanabhan said: “REGENETEN is the first solution of its kind to treat large and massive thickness rotator cuff tears. More than 650,000 rotator cuff procedures take place annually in the US, potentially growing at a rate of 5-6% each year.
“This data reinforces our confidence that this revolutionary technology is truly a game-changer in the treatment of patients with rotator cuff disease.”
According to the company, repairs of large and massive rotator cuff tears are known to have a high rate of failure, with more than 40% cases require further treatment.
The REGENETEN implant stimulates the body’s natural healing response
The collagen-based implant has been designed to stimulate the body’s natural healing response. It induces the growth of new tendon-like tissue to biologically augment the existing tendon and prevent the disease progression.
The REGENETEN implant, approximately in size of a postage stamp, is delivered arthroscopically through a small incision over the location of the rotator cuff tendon injury, which is gradually absorbed within 6 months, said the company.
Consecutive ultrasound examinations up to 24 months and a single post-operative MRI were conducted to assess the tendon healing. The results showed an increased tendon thickness from 3 months to 12 months before slightly decreasing at 24 months, indicating functional remodelling of the new tissue.
Furthermore, the efficacy of the implant in patients with partial-thickness and small full-thickness tears is well-known with data showing rapid and sustained healing. The implant helped in reducing the tear size in 94% of partial-thickness tear patients and prevented re-tears in small full-thickness tears.
The study of REGENETEN Bioinductive Implant, which is currently available in the US, was published in the American Journal of Sports Medicine. The study reported no implant-related adverse events, and no significant difference in treatment success between primary repairs and revision surgery.
