The US-based start-up, which is engaged in developing high data-rate brain-computer interface, plans to use the funds to launch the first-in-human clinical trial for Connexus DDI
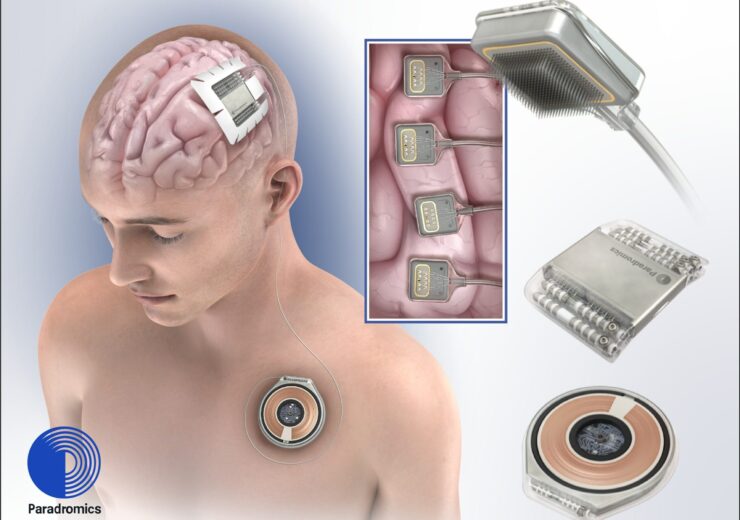
Connexus DDI translates brain signals into speech and movement in real-time. (Credit: PR Newswire/Paradromics)
Paradromics has secured $33m in a Series A funding round apart from getting the breakthrough device designation for its Connexus Direct Data Interface (DDI) implant from the US Food and Drug Administration (FDA).
The US-based start-up is engaged in developing high data-rate brain-computer interface (BCI). It plans to use the Series A proceeds to launch the first-in-human clinical trial for the Connexus DDI implant.
The Series A round was led by Prime Movers Lab and included additional investors like Westcott Investment Group, Green Sands Equity, and Dolby Family Ventures.
According to Paradromics, the first application of the Connexus DDI is a BCI-enabled assistive communication device for severely motor-impaired patients.
Connexus DDI translates brain signals into speech and movement in real time for people with conditions like amyotrophic lateral sclerosis (ALS), spinal cord injury, and stroke.
The assistive communication device restores social connection and enables independent engagement with technology, the BCI developer claimed. It is said to have the potential to transform the treatment of neurological and brain-related conditions.
According to Paradromics CEO Matt Angle, the FDA breakthrough status and the latest funding are expected to support the company’s path to market launch.
Angle said: “This designation recognises the transformative promise of our device, and we look forward to continued coordination with the FDA to accelerate its availability.
“And this investment validates our leadership position among the small group of BCI platform companies on the verge of commercialisation.”
Apart from assistive communication, the brain-computer interface technology can address various unmet medical needs, ranging from motor and sensory deficits to chronic pain and mood disorders.
Paradromics said that the FDA breakthrough device designation will allow an expedited review process for the Connexus DDI.
Prime Movers Lab founder and general partner Dakin Sloss said: “Brain computer interfaces will transform mental health treatments, making it an exciting investment opportunity.
“We’re seeing only a couple of companies emerge as real contenders in the space, and I believe Paradromics will be the one that moves into successful human trials.”
In July 2021, the company raised $20m in a seed funding round, which was also led by Prime Movers Lab.
