FDA STeP Program awarded to OtoNexus Medical Technologies for ultrasound platform technology that evaluates middle ear infections
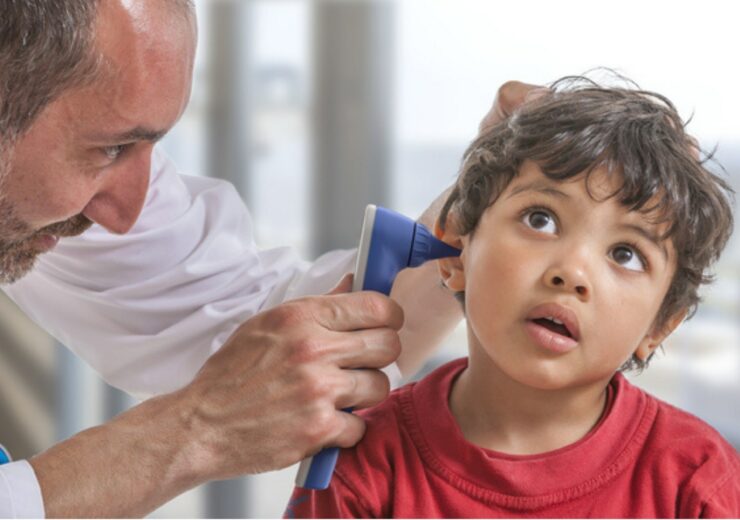
OtoNexus Medical Technologies has developed a novel ultrasound otoscope for the immediate and automatic differentiation of bacterial middle ear infections. (Credit: PR Newswire/ OtoNexus Medical Technologies Inc.)
OtoNexus Medical Technologies, the developer of the Novoscope ultrasound otoscope for immediate and automatic differentiation of bacterial middle ear infections, is excited to announce its recent selection into U. S. Food and Drug Administration Safter Technologies Program (STeP).
STeP is a pioneering initiative introduced by the FDA to identify and expedite the development and review of innovative medical devices that offer a significant advantage over all currently available options, in that devices selected for the program provide radical improvements in patient care and patient safety.
STeP aims to provide patients with more timely access to these medical devices by providing STeP awardees expedited development and review, and interactive and timely communications, including sprint discussions, enhanced regulatory support, real-time feedback and direct advice, and other support. To qualify, the product must offer a significant advantage over currently available options.
The evaluation process for the program requires comprehensive due diligence by the FDA to ensure the technology meets the strict, extensive criteria. “We are truly honored to be chosen for inclusion in the Safer Technologies Program,” stated Caitlin Cameron, Chair and CEO of OtoNexus Medical Technologies. “This program provides a unique opportunity to collaborate closely with the FDA, ensuring that our technology attains the highest levels of safety and efficacy for market clearance with a faster development and review timeline, with a joint goal with the FDA of significantly improving patient safety and patient care.”
In 2022, just 14 devices were selected for the elite Safer Technologies Program, an innovative initiative of the Center for Devices and Radiological Health (CDRH) division of the FDA designed to identify and expedite the development of devices expected to significantly improve safety, treatment, and diagnostics. CDRH oversees 238,000 regulated medical devices and 27,000 device manufacturing firms and received 18,000 total submissions.
Source: Company Press Release
