MRIdian A3i includes a suite of new features aimed at enhancing on-table adaptive workflow, enabling clinicians to auto-contour, auto-adapt, and auto-gate, intelligently
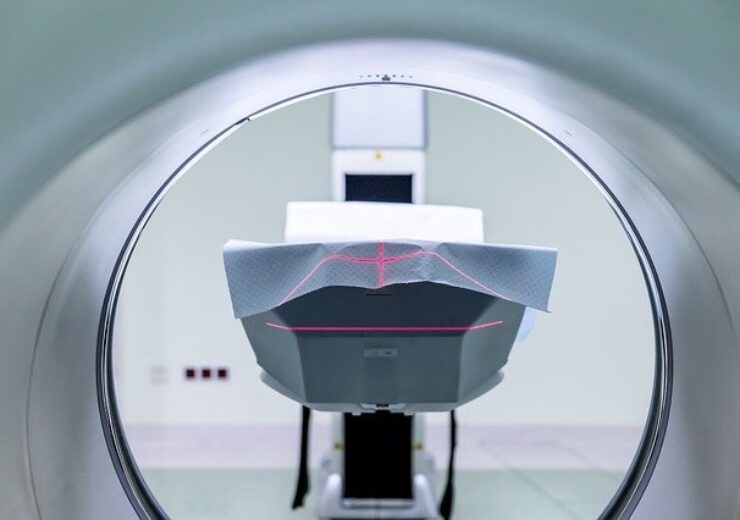
MRIdian system offers advanced anatomical visualisation. (Credit: Michal Jarmoluk from Pixabay.)
ViewRay has secured the US Food and Drug Administration (FDA) 510(k) clearance for its updated version of MRIdian MRI-guided radiation therapy system.
MRIdian A3i includes a suite of new features aimed at enhancing on-table adaptive workflow, enabling clinicians to auto-contour, auto-adapt, and auto-gate, intelligently.
It comes with a new brain treatment package that expands clinical utility into cranial stereotactic radiosurgery (SRS) and stereotactic radiation therapy (SRT).
The automated workflow tools reduce treatment times, increase patient throughput, and allow clinicians to connect remotely during patient treatment, said the company.
ViewRay president and CEO Scott Drake said: “As the only provider of a commercially available MR-guided technology with real-time tissue tracking and automatic beam gating technology, we are proud to build on our unique offering with the next generation of MRIdian.
“MRIdian A3i addresses our customers’ desire for faster treatment times, increased patient throughput and the ability to expand their programs and offer MRIdian SMART to a larger patient population.”
MRIdian A3i is said to expand the existing real-time tissue tracking and automated beam gating features into multiplanar tracking and gating, in up to three planes.
It enables the customers to select up to three different tracking targets to stop the beam when any single target exceeds the treatment boundaries.
Also, the system comes with an integrated patient breath-hold display, to help the patient keep their tumour in an optimal treatment position for effective radiation.
The new brain treatment package comprises a dedicated brain coil with an integrated stereotactic brain immobilisation system.
Its new, high-resolution volumetric and real-time imaging features enable the treatment of brain metastases, resection cavities, gliomas, and other cranial lesions.
According to ViewRay, its MRIdian system provides advanced anatomical visualisation to oncologists, through diagnostic-quality MR images.
The system was used to treat more than 16,000 patients, and 46 MRIdian systems are currently in clinical use at hospitals worldwide, to treat different types of solid tumours.
Furthermore, MRIdian Linac System is not recommended for patients who are not candidates for MRI, as radiation may cause side effects.
