In a trial, the EyeArt v2.2.0 system showed 91.1% specificity and 94.4% sensitivity for more than mild DR detection, along with 96.8% sensitivity and 91.6% specificity for vision-threatening DR detection
The EyeArt system from Eyenuk. (Credit: GlobeNewswire/Eyenuk, Inc.)
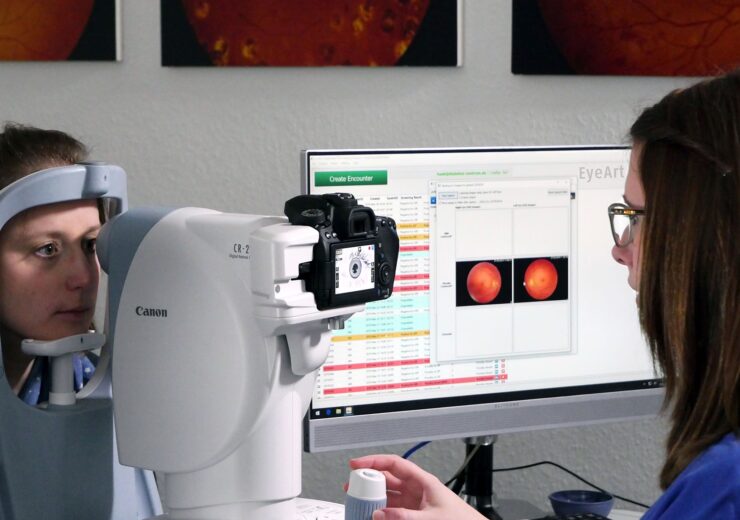
Eyenuk has received clearance from the US Food and Drug Administration (FDA) to use the Topcon NW400 retinal camera with its EyeArt AI system for autonomous artificial intelligence (AI)-based detection of diabetic retinopathy (DR).
The latest regulatory milestone adds to the EyeArt AI system’s existing clearance for use with Canon CR-2 AF and Canon CR-2 Plus AF cameras.
The EyeArt v2.2.0 system is now said to be the first and only FDA-cleared AI system cleared for use with multiple retinal cameras by various manufacturers.
The recent FDA nod follows the system’s launch in August 2020 as the first and only FDA-cleared AI technology to identify diabetic retinopathy that is more severe than mild and vision-threatening.
Eyenuk, a US-based digital health company, said that it is also the first and only AI system authorised under MDR Class IIb in the European Union to identify glaucomatous optic nerve damage, age-related macular degeneration, and diabetic retinopathy in a single test.
Since its launch in the global market, the EyeArt system has been used for safe and reliable eye screening for more than 230,000 patients with diabetes.
Eyenuk founder and CEO Kaushal Solanki said: “I am particularly thrilled about this clearance as it showcases our extraordinary commitment towards our mission to screen every eye in the world to ensure timely diagnosis of life- and vision-threatening diseases.
“The EyeArt system can now be used with multiple camera models in the US, which significantly expands access for patients who can be screened in their primary care doctor’s office for preventable blindness due to diabetes.”
The US health regulator cleared the EyeArt v2.2.0 system based on clinical data from a prospective multi-centre clinical trial.
In the trial, the system showed 91.1% specificity and 94.4% sensitivity for more than mild diabetic retinopathy detection. For vision-threatening diabetic retinopathy detection, the system delivered 96.8% sensitivity and 91.6% specificity.
In addition, the EyeArt system received clearance from the FDA for an updated image quality assessment module and the proprietary real-time image quality feedback solution of Eyenuk.
The digital health firm said that these upgrades increase the usability of the EyeArt system, achieving the best-in-class gradability without dilation.
