TRICORDER, an implementation programme funded National Institute for Health and Care Research (NIHR), and led by researchers at Imperial College London, will deploy Eko’s AI-powered technology in primary care practices across the UK
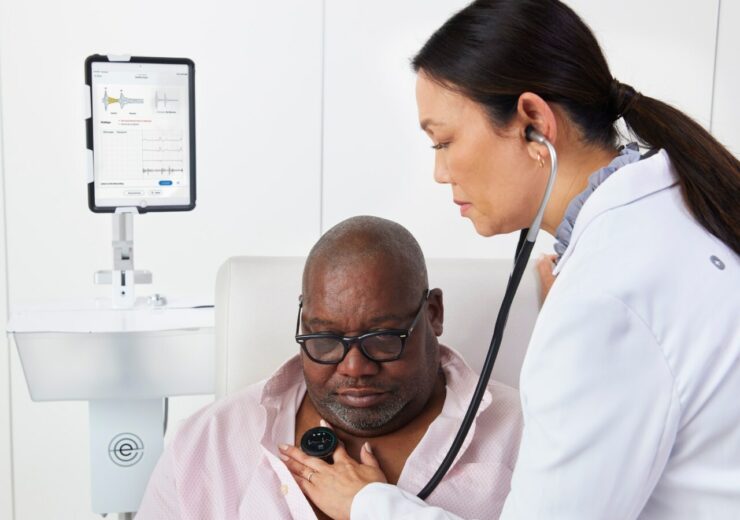
Eko Health’s AI-enabled technology marketed as SENSORA in the US. (Credit: PRNewswire/Eko Health)
US-based healthcare technology company Eko Health has received the UKCA marking approval and is deploying its AI-enabled heart disease detection technology across the UK.
TRICORDER, an implementation programme led by researchers at Imperial College London, will deploy Eko’s AI-powered technology in primary care practices across the UK.
Eko’s AI-powered technology, offered as SENSORA in the US, helps clinicians detect heart failure (HF), valvular heart disease (VHD), and atrial fibrillation (AFib).
It combines a digital stethoscope, electrocardiogram (ECG) and advanced machine learning algorithms to analyse ECG and heart sound data.
Funded National Institute for Health and Care Research (NIHR), TRICORDER will enable the deployment of Eko’s AI technology to 100 General Practitioner (GP) practices across the UK.
The patients visiting their GP will receive a brief, non-invasive cardiac exam, which leverages an Eko digital stethoscope and app powered by its AI technology.
When the AI detects signs associated with cardiac disease, the GP can rapidly initiate further tests and potentially start life-saving treatment.
Eko said the deployment of its AI technology would improve heart disease outcomes for people in different communities across the UK.
Eko Health co-founder and CEO Connor Landgraf said: “This deployment around the UK of our AI-enabled heart disease detection technology demonstrates the need for accurate and early heart disease detection.
“We’re proud to partner with leading academic institutions such as Imperial College London to validate the clinical utility and positive impact our technology has on the lives of millions of patients.”
Eko said its HFrEF algorithm has been independently validated, in which the algorithm achieved around 80% sensitivity and specificity in detecting HFrEF.
The company has submitted the HFrEF detection algorithm to the US FDA seeking 510(k) approval, with plans to market the SENSORA in the US, after receiving the regulatory approval.
Its structural murmur algorithm detected valvular heart disease with more than double the accuracy compared to the standard method by primary care professionals in a validation study.
Furthermore, the US healthcare technology company said that its AFib detection algorithm has been clinically validated to demonstrate 100% sensitivity and 96.2% specificity.
