The advanced AI-powered imaging solution is designed to help dentists detect common dental conditions from dental x-rays, including caries, periapical lesions, and bone loss
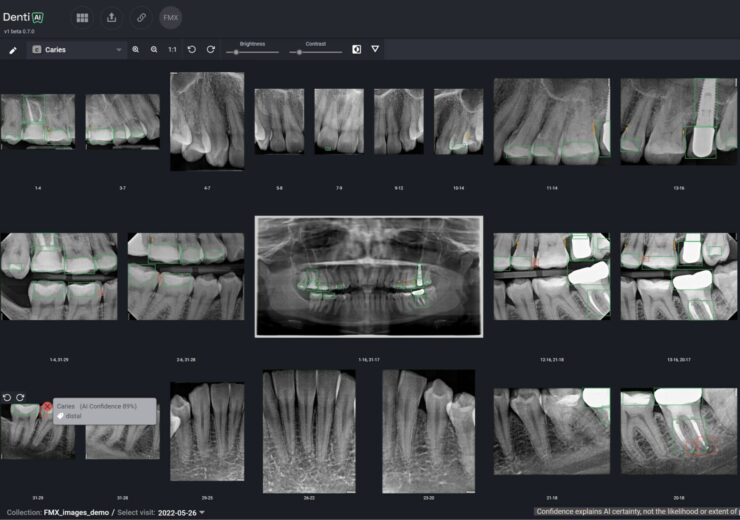
An interface of combined Denti.AI Detect and Auto-Chart features. (Credit: PRNewsfoto/Denti.AI)
Canada-based Denti.AI has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Denti.AI Detect product for pathology detection in both panoramic and intraoral X-rays.
Denti.AI Detect is said to be an advanced artificial intelligence (AI)-powered imaging solution. It has been designed to help dentists detect common dental conditions from dental x-rays, including caries, periapical lesions, and bone loss.
Denti.AI, which has expertise in AI-based dental pathology detection, odontogram creation, and periodontal charting automation, said that clinical trials have shown that Denti.AI Detect has opened up 26% more treatment options.
Two dozen participating dentists who took part in the trials are said to have significantly improved their illness diagnosis.
The latest FDA clearance follows the authorisation of Denti.AI Auto-Chart in November 2022. Denti.AI Auto-Chart is claimed by the company to be a breakthrough in automating odontogram charting of restorations, root canals, implants, crowns, and missing teeth.
Denti.AI Detect has been integrated with Auto-Chart to further improve disease charting and treatment plan coding in various integrated practice management systems (PMS). The integration represents a major advancement in improving patient care and streamlining dental practice workflows, the dental technology provider said.
Denti.AI founder and CEO Dmitry Tuzoff said: “The realisation of Denti.AI Detect clearance signifies countless hours of dedication from our team.
“The introduction of panoramic imaging technology, especially, propels our product to the forefront, addressing the growing usage of such imaging practices across North America.”
The FDA clearance is also the industry-first such for disease detection on panoramic images, said the company.
Denti.AI has now become the only dental AI firm to receive clearance for all three primary dental concerns, which are caries, periapical radiolucencies, and bone levels.
Prior to receiving FDA clearance, Denti.AI Detect had already obtained approval from Health Canada and is said to be widely utilised in numerous dental practices throughout Canada.
