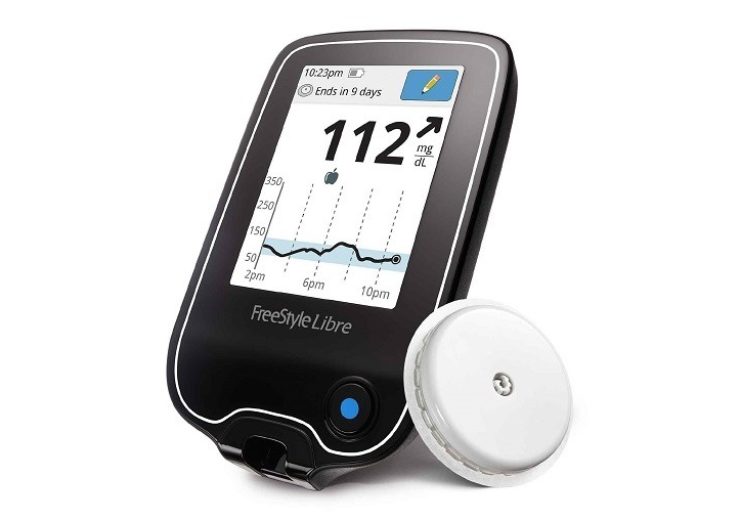
The collaboration will integrate Abbott’s FreeStyle Libre sensor with Insulet’s Omnipod Horizon Automated Insulin Delivery (AID) System
Abbott, Insulet collaborate on integrated solution for diabetes. (Credit: Abbott.)
US-based medical devices firm Abbott has joined forces with Insulet, focused on medical devices for diabetes, to offer personalised automated insulin delivery and care for diabetics.
Under the collaboration, Abbott’s FreeStyle Libre sensor will be integrated with Insulet’s Omnipod Horizon Automated Insulin Delivery (AID) System.
The integrated platform, comprising Insulet’s tubeless insulin delivery pod, and Abbott’s advanced glucose sensing technology, can be controlled through an app on a user’s personal smartphone device.
Abbott diabetes care senior vice president Jared Watkin said: “Abbott is focused on creating future-forward health technologies that simplify how people living with diabetes manage their condition so they can live their best lives.
“As diabetes care becomes more interoperable, we’re developing more connected approaches to improve care. Through this partnership, Abbott and Insulet will offer an integrated digital health platform that is simple and accurate and will provide a best-in-class user experience.”
The integrated platform will integrate Omnipod Horizon System and FreeStyle Libre sensor
The platform with simple design will capture the glucose data from the sensor and sends directly to the Pod that is embedded with an algorithm to automatically adjust insulin delivery, preventing the need for an additional device, connection or tubing.
In addition, the integrated platform is designed to be in an automated insulin delivery mode, enabling users to manually control the dosing of fast-acting insulin, through a smartphone app, for optimal performance.
The combined diabetes solution will integrate data from both the Omnipod Horizon System and FreeStyle Libre sensor, and offers an effective option for people with diabetes.
Seperately, Abbott secured CE mark approval for its new Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) devices.
With an advanced patient app and Bluetooth connectivity capabilities, the new devices offer improved heart rhythm management capabilities.
