Designed and developed by the OIC, the Bone Bolt System is an implant system for percutaneous bone fracture fixation including pelvic fractures and fractures of the long bones in the arm and leg
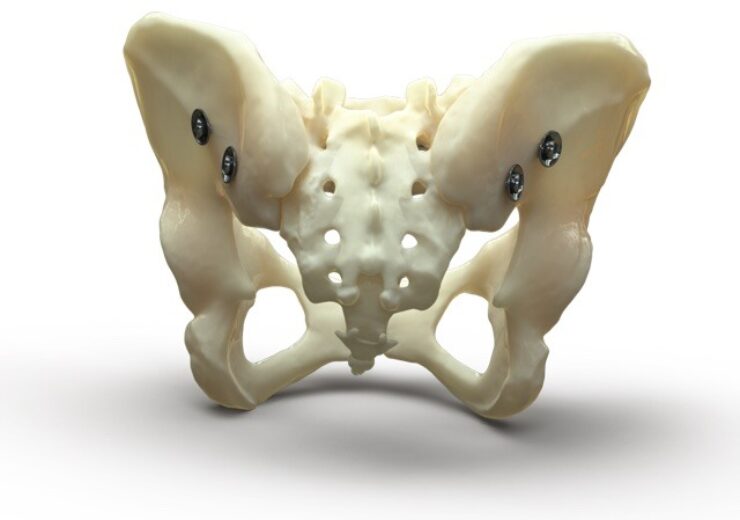
The Bone Bolt System during a posterior pelvic fracture repair procedure. (Credit: University of Utah Health)
The University of Utah Orthopaedic Innovation Center (OIC), Department of Orthopaedics and Spencer Fox Eccles School of Medicine has secured the US Food and Drug Administration (FDA) 510(k) clearance for its Bone Bolt System to treat bone fractures.
The clearance will enable the Bone Bolt System to be sold and advertised in the US.
The medical device is said to be the first in the history of the University of Utah to receive approval from the FDA under 510(k) regulations.
Designed and developed by the OIC, the Bone Bolt System is an implant system for percutaneous bone fracture fixation.
The system consists of a wide range of implants with different lengths and diameters and related surgical tools and sterilisation trays. These are used to treat difficult bone fractures, such as pelvic fractures and fractures of the long bones in the arm and leg.
The Bone Bolt System is covered by a US patent, with additional domestic and foreign patent applications pending.
University of Utah president Taylor Randall said: “As one of the nation’s leading research universities, innovation plays a central role in our mission to improve the quality of life of our patients—OIC’s Bone Bolt System does just that.
“Not only will the device make a difference in the care we can provide at University of Utah Health, but it illustrates our commitment to turn research into real-world impact by getting it to market as soon as possible, to improve health outcomes more broadly.”
The University of Utah’s OIC submitted a 510(k) to the US health regulator to validate that the device is safe, effective, or substantially equivalent, to an existing FDA 510(k) cleared device.
The OIC is said to have created the Bone Bolt System in complete adherence to the ISO 13485 Medical Devices—Quality Management Systems and FDA Quality System Regulations. It also created a commercial supply chain for the system.
OIC and the University of Utah PIVOT Center will now aim to set up industry partnerships to market the Bone Bolt System and bring it to hospitals and surgery centres across the US to help orthopaedic patients.
