Orders will be processed immediately and shipments will start in the coming weeks
Medtronic will begin processing orders immediately and will start shipments over the next few weeks to those with type 1 diabetes who meet eligibility criteria. (Credit: Medtronic)
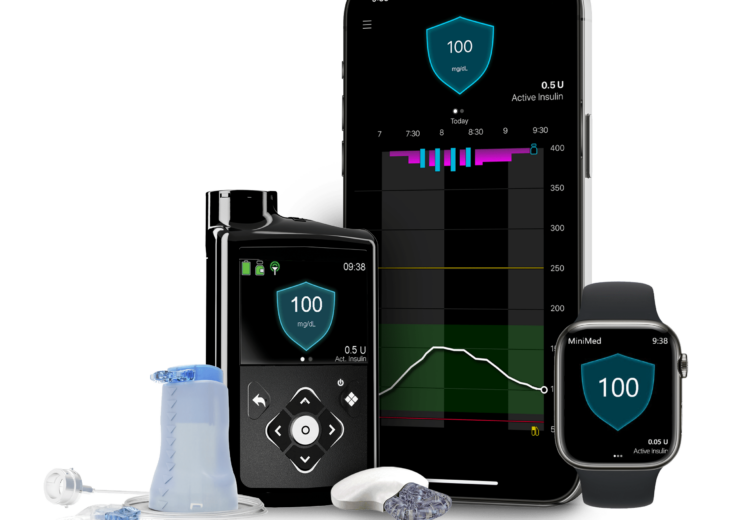
Medtronic, a global leader in healthcare technology, announced today that its MiniMed 780G system with the new Guardian 4 sensor requiring no fingersticks while in SmartGuard technology is now covered for all eligible Medicare and Medicare Advantage beneficiaries.
Medtronic will begin processing orders immediately and will start shipments over the next few weeks to those with type 1 diabetes who meet eligibility criteria.
“Coming off the heels of a very exciting 83rd American Diabetes Association Scientific Sessions where we shared the latest real-world data on our MiniMed 780G system, we couldn’t be more thrilled to expand access to our Meal Detection technology that’s helping many individuals around the world achieve an average Time in Range exceeding 80 percent when using recommended settings,” said Que Dallara, EVP and President, Medtronic Diabetes. “We see people of all ages and from different therapies benefitting significantly from improved outcomes with the MiniMed 780G system. We attribute this to the easy-to-use design coupled with a powerful algorithm that provides coverage when life gets in the way — one that’s designed for real life.”
The MiniMed 780G system delivers automatic adjustments and corrections† to sugar levels every 5 minutes to provide coverage for when users occasionally forget to bolus or underestimate the number of carbs in their meal.
It features the lowest target setting (as low as 100 mg/dL) in any automated insulin pump on the market to more closely mirror the average glucose of someone not living with diabetes.
“What’s particularly compelling about the MiniMed 780G system is that it broadens eligibility to many more individuals interested in automated insulin delivery to help them achieve their diabetes management goals,” said Robert Vigersky, MD, Chief Medical Officer, Medtronic Diabetes. “Historically, there’s been a stepwise approach towards prescribing pump therapy when certain criteria were met including the ability to carb count and use advanced technology. This approach is no longer relevant given the system’s ability to overcome these challenges. We are very pleased that we’re able to make this technology available to Medicare beneficiaries.”
Source: Company Press Release
