The TiLink-L SI joint fusion system is designed to be implanted from a lateral or posterior approach and leverages the company’s unique Nanotex surface technology to optimise bone growth
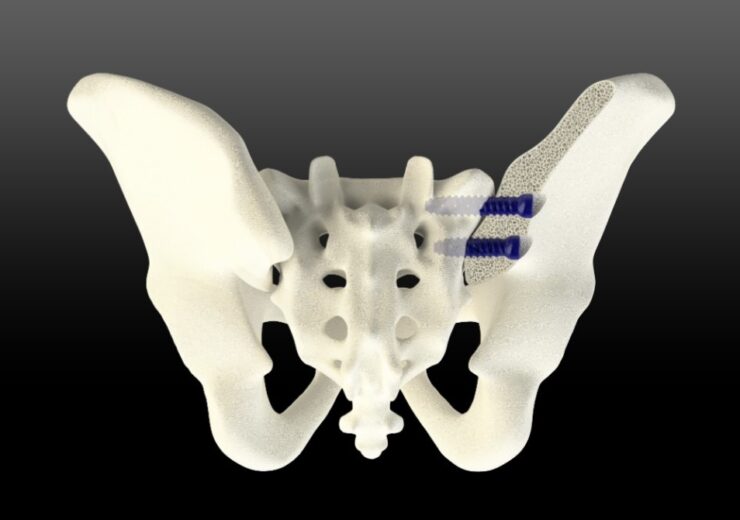
TiLink-L SI joint fusion system in lateral approach. (Credit: Business Wire/ SurGenTec)
US-based medical device company SurGenTec has received the US Food and Drug Administration (FDA) approval for its TiLink-L Sacroiliac (SI) joint fusion system.
The TiLink-L system is designed to be implanted from a lateral or posterior/oblique approach and leverages surface technology to optimise bone growth.
The SI joint fusion implant features the company’s Nanotex surface technology.
Also, it comes with an intuitive design that allows superior compression across the joint and provides an outstanding platform for fusion.
SurGenTec said that TiLink-L is the first implant in its Sacroiliac family of products.
SurGenTec CEO Travis Greenhalgh said: “We are thrilled to announce the FDA clearance of our first treatment option in our sacroiliac family of products.
“With its unique properties and ability to adapt to diverse patient needs, TiLink-L is set to offer physicians the ability to stabilise the sacroiliac joint in a variety of approaches.
“Whether in an outpatient or hospital setting, we believe this device is poised to make a significant difference in patient care.”
In the in-vivo studies, conducted by a Good Laboratory Practice Facility, the use of TiLink-L implants showed a bony in-growth and on-growth, indicating improved surgical outcomes.
Also, the implant is said to create a helical self-harvesting channel to capture the patient’s own bone and encourage healing.
SurGenTec said that its graft windows are strategically placed between threads, to facilitate potential fusion through the screw to enhance stability.
It is offered in different implant lengths, to complement the essential features, enable customised fitting, optimal fixation, and reduce potential complications.
According to the company, the demand for minimally invasive surgical solutions increases with the ageing of the population, and TiLink-L is strategically positioned to address this need.
SurGenTec is a medical device company focused on developing and manufacturing medical devices and technologies to improve orthopaedic and spine care.
The company plans to immediately commercialise the implant in the US.
