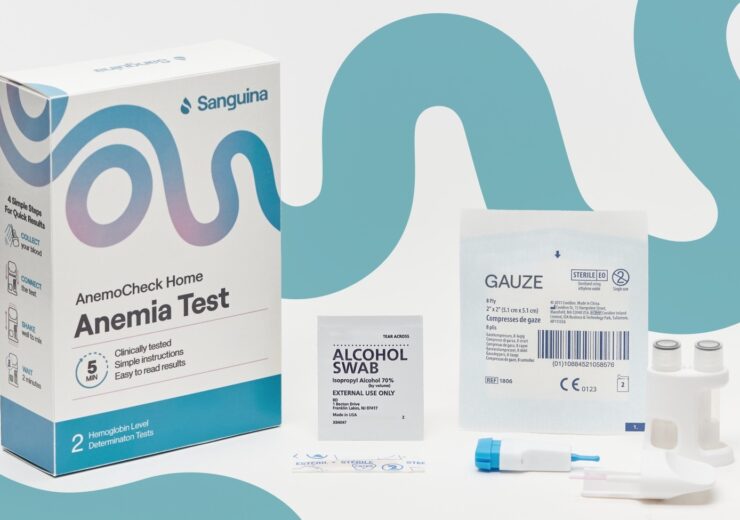
AnemoCheck Home is a disposable, in-vitro diagnostic device, designed to help individuals with anaemia due to nutritional deficiency, sickle cell disease and thalassemia, monitor their haemoglobin levels at their home
AnemoCheck Home test provides instant results. (Credit: Business Wire)
US-based wellness and diagnostic company Sanguina has received the US Food and Drug Administration (FDA) approval for its at-home haemoglobin test kit, AnemoCheck Home.
AnemoCheck Home is an advanced solution for detecting and monitoring anaemia, a condition characterised by the shortage of healthy red blood cells or haemoglobin.
It is designed as an in-vitro diagnostic device to help individuals with anaemia due to nutritional deficiency, sickle cell disease and thalassemia, monitor their haemoglobin levels at their home.
The FDA-approved at-home test allows people with anaemia to obtain accurate measurements of haemoglobin levels, through a simple fingerstick blood test.
Sanguina CEO Erika Tyburski said: “Our team at Sanguina is proud to introduce AnemoCheck Home as a game-changer for at-home anaemia testing.
“With this FDA clearance, we are excited to provide people who have anaemia with a convenient, accurate, and accessible tool to monitor their haemoglobin levels at home.”
Sanguina said that its AnemoCheck Home leverages the latest advancements in healthcare technology to provide accurate results to help users effectively manage their anaemia.
The test kit is offered only through prescription and comprises user instructions and all other equipment required to perform the fingerstick test safely.
Users need to perform a finger stick, collect the blood into a sample collection tube, and then connect the test cap with a test body, and shake it to mix the blood.
After two minutes, the disposable test changes its colour which can be correlated to a haemoglobin level on a colour card.
Established in 2019, Sanguina is engaged in providing advanced digital and at-home solutions for diagnosing, monitoring, and managing wellness and chronic conditions.
In 2021, the biotech company relaunched its AnemoCheck Mobile App with an updated design, new capabilities, and additional content.
