The new ultrasound solution integrates advanced transducers, processing, and software tools to optimise the paediatric diagnostic workflow
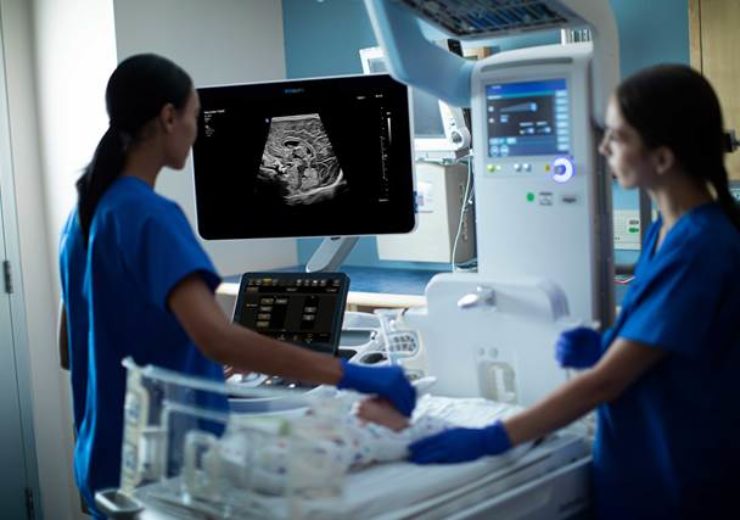
Philips ultimate ultrasound solution for paediatric assessment. (Credit: Koninklijke Philips N.V.)
Health technology company Royal Philips has introduced a new ultimate ultrasound solution for paediatric assessment.
Part of the Philips Ultrasound System (EPIQ Elite) platform, the new ultimate ultrasound solution offers clinicians with comprehensive images and the required performance to make a precise diagnosis for paediatric patients.
Designed specifically for paediatric clinical use, the new ultrasound solution integrates transducers, processing, and software tools that are enhanced for the paediatric diagnostic workflow.
Philips provides a complete imaging solution to optimise diagnostic confidence for paediatric patients with different shapes and sizes ranging from the tiniest premature newborn baby to adult-sized paediatric patients.
The new ultrasound solution offers tailored paediatric workflow to help clinicians diagnose paediatric patients more rapidly
Cleared for paediatric clinical use, the ultrasound solution features a new paediatric transducer that helps to offer 30% more penetration for complete images. It also offers a tailored paediatric workflow to enable clinicians diagnose paediatric patients more rapidly.
Philips offers ultrasound solutions for the evaluation of small parts, liver, vascular, breast, in addition to paediatrics.
Philips general imaging ultrasound general manager and vice president Jeff Cohen said: “Diagnostic confidence is vital for any clinician, especially when diagnosing the most sensitive patients.
“Philips ultimate ultrasound solutions provide the right level of image detail and context that clinicians need to make a precise diagnosis.”
In April this year, Philips introduced new Respironics E30 ventilator, a new emergency use ventilator with visual and audible alarms for COVID-19 patients.
The company has secured emergency use authorisation (EUA) for the new ventilator to address the COVID-19 public emergency.
