Additionally, analysis of two-year physiological range of motion data from multiple artificial cervical disc IDE studies to be presented showing benefits of the nucleus-annulus design of the M6-C disc
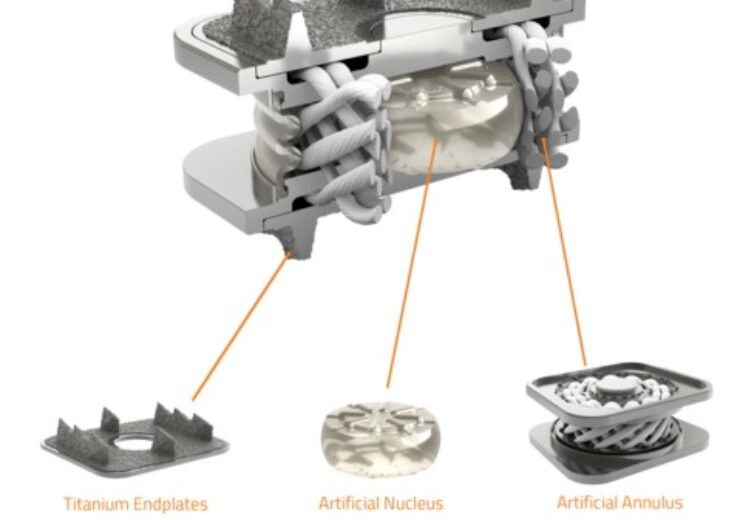
Cutaway image of the M6-C artificial cervical disc showing the unique nucleus-annulus design that mimics the natural structure of a native disc. (Credit: Business Wire/ )
Orthofix Medical, a global medical device company with a spine and orthopedics focus, today announced the presentation of five-year data from the M6-C artificial cervical disc single-level IDE study. These results will be presented on Friday, June 3 during the International Society for the Advancement of Spine Surgery (ISASS) annual meeting at The Atlantis Hotel in Nassau, Bahamas.
“We are pleased to see that at five years postoperative, participants in the study who received the M6-C artificial cervical disc continue to show significant benefits in Neck Disability Index (NDI) scores and neck and shoulder/arm pain Visual Analog Scale (VAS) scores compared to patients who received anterior cervical discectomy and fusion (ACDF),” said Dr. Frank Phillips, Professor of Orthopedic Surgery at Rush University Medical Center and an investigator in the study, who will present the data at the ISASS meeting. “Additionally, M6-C patients in the study had a low rate of revision surgery compared to the ACDF cohort. Patients in the study will be monitored out to 10 years post-op as we continue to compile the data supporting the efficacy and benefits of cervical disc arthroplasty with the M6-C disc.”
“Mobility and stability are two essential requirements which allow a spinal segment to function in harmony with its neighboring segments,” said Dr. Avinash Patwardhan. “The M6-C disc, with its inherent progressive resistance to motion provided by the nucleus-annulus design, yielded the highest proportion (103/131 or 79 percent) of implanted segments in the physiological motion range compared to the cohort average of 65 percent. These results support the functional goals of CDA which include restoring segmental range of motion and reducing the risk of accelerated adjacent segment degeneration.”
“We continue to be pleased with the U.S. IDE clinical data that now includes five-year outcomes,” said Orthofix President of Global Spine Kevin Kenny. “We recognize the importance of the ongoing collection of data from the U.S. study and implanting centers around the globe in order to expand the body of evidence that supports the clinical performance and safety of the M6-C artificial cervical disc. We look forward to continuing to disseminate and publish that data.”
Source: Company Press Release
