The Lunit INSIGHT DBT is an AI-powered medical device designed to analyse images generated by 3D Breast Tomosynthesis (DBT) equipment, which provides a rapid and accurate breast cancer diagnosis compared to traditional 2D mammography screenings
Lunit’s AI-powered 3D Breast Tomosynthesis analysis solution. (Credit: PRNewswire/ Lunit)
South Korea-based AI-based cancer diagnostics provider Lunit has received the US Food and Drug Administration (FDA) 510(k) approval for its Lunit INSIGHT DBT solution.
The Lunit INSIGHT DBT is an artificial intelligence (AI)-powered medical device, designed to analyse images generated by 3D Breast Tomosynthesis (DBT) equipment.
According to the medical AI company, DBT imaging provides a rapid and accurate breast cancer diagnosis compared to traditional 2D mammography screenings.
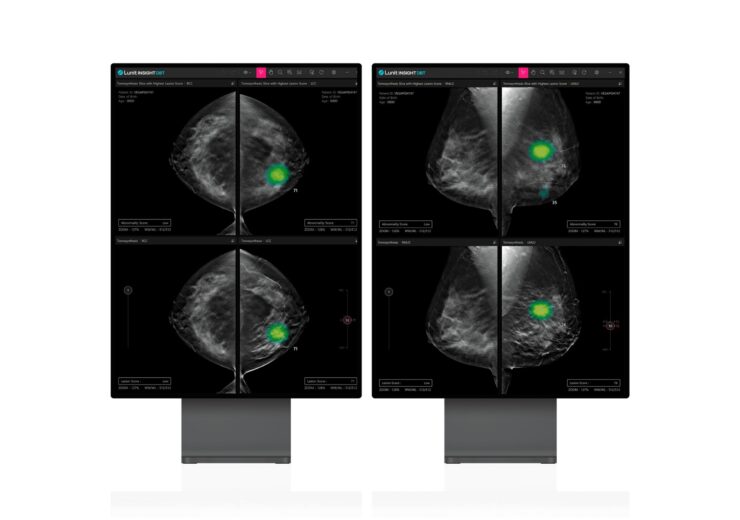
The AI-powered DBT tool quantifies the chances of malignancy for each suspicious lesion with an abnormality score and displays location information with heatmap or contours.
It also provides information on lesion types, such as soft tissue lesions with mass, architectural distortion, and asymmetry and calcification.
Also, Lunit INSIGHT DBT highlights each lesion for enhanced visibility and presents the best 3D slice to show a suspicious lesion which advances the tomo reading.
Lunit CEO Brandon Suh said: “The US is the biggest player in the global breast screening market, accounting for up to 40% of the market share. More than 40 million mammography screenings are reported in the US annually.
“Given this substantial market influence, achieving FDA clearance for Lunit INSIGHT DBT not only solidifies our presence in the largest market but also marks a significant milestone in our mission to revolutionise breast cancer diagnosis and, ultimately, save more lives.”
The Lunit INSIGHT DBT has already been approved in Europe since March 2023, with a CE mark under Europe’s updated Medical Device Regulation (EU MDR).
With the FDA approval, the company aims to bring advanced AI breast imaging to cancer diagnosis, making early detection and treatment of breast cancer more accessible and accurate.
Also, the medical diagnostics company is enabled to enter the US breast screening market.
Established in 2013, Lunit is focused on developing AI solutions for precision diagnostics and therapeutics, to ensure the right diagnosis and treatment, at the right cost.
The company develops advanced medical image analytics and AI-based biomarkers leveraging advanced technology and its application in medical images.
The FDA approval of Lunit INSIGHT DBT follows its chest X-ray triage solution, Lunit INSIGHT CXR Triage, and AI-powered mammography analysis solution Lunit INSIGHT MMG.
