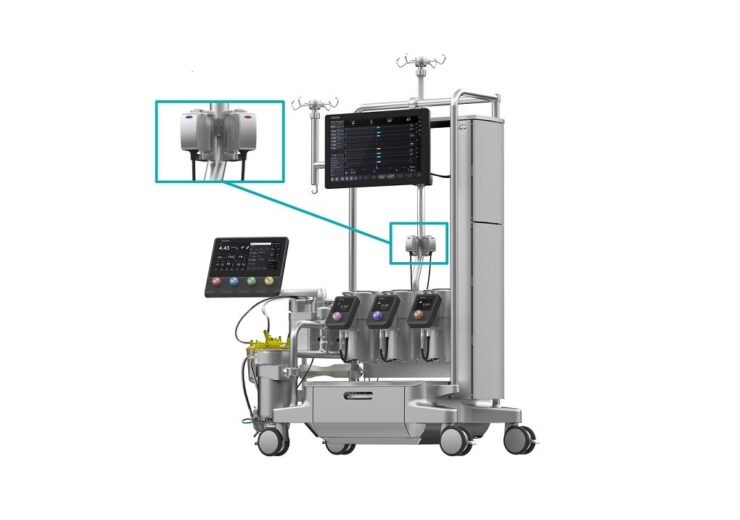
Integrated into LivaNova’s Essenz Perfusion System, Essenz ILMB is designed to provide perfusionists with accurate and continuous measurement of essential blood parameters throughout cardiopulmonary bypass (CPB) procedures
Essenz In-Line Blood Monitor. (Credit: Business Wire)
UK-based medical technology company LivaNova has received the US Food and Drug Administration (FDA) 510(k) approval and CE Mark for its Essenz In-Line Blood Monitor (ILBM).
Essenz ILMB is designed to provide perfusionists with accurate and continuous measurement of essential blood parameters throughout cardiopulmonary bypass (CPB) procedures.
It is integrated into LivaNova’s Essenz Perfusion System, which allows perfusionists to manage blood parameters directly from the system’s cockpit, eliminating additional monitors.
The Essenz ILBM is powered by LivaNova’s B-Capta sensing technology, to monitor arterial and venous blood gas parameters in cardiopulmonary bypass procedures.
It provides measured values for oxygen saturation, haematocrit, partial pressure of oxygen and temperature, instead of calculated values for the parameters.
Also, Essenz ILBM does not require any calibration to adjust the device measurements, which allows the perfusionist to save time during device set-up, especially in emergency cases.
Essenz ILBM is the only in-line blood monitoring system that works as per the Clinical Laboratory Improvement Amendments (CLIA) guidelines and provides parameter values in line with hospital blood gas analysers, said the company.
LivaNova cardiopulmonary president Marco Dolci said “Dynamic conditions can rapidly change a patient’s blood parameters during a cardiopulmonary bypass procedure.
“The Essenz In-Line Blood Monitor provides continuous monitoring throughout a patient’s procedure.
“Access to accurate, real-time measurements directly from the Essenz Perfusion System allows for quick decisions and tailored care strategies to serve the patient.”
According to the company, existing blood gas analysers reflect the patient’s clinical condition only at the time of sample collection, which may rapidly change and become irrelevant.
Its Essenz ILBM provides perfusionists with in-line continuous monitoring of the patient’s parameters for the entire duration of a procedure, enabling data-driven, patient-specific decisions.
The device automatically transfers arterial and venous parameters to the Essenz Patient Monitor, supporting goal-directed perfusion (GDP) therapy.
The Essenz Perfusion System comprises a next-generation heart-lung machine (HLM), a patient monitor and accurate sensing technology that includes the ILBM.
LivaNova said that its latest heart-lung machine software, version 1.3, integrates the ILBM with the Essenz Perfusion System and enhances the user experience.
