The advanced test will facilitate rapid, easy, and comfortable larger-scale testing, and provides accurate identification in less than five minutes
Imec begins developing new SARS-CoV-2 test. (Credit: PRNewsfoto/Imec.)
Imec, a nanoelectronics and digital technologies provider, has announced its plans to begin development of an advanced SARS-CoV-2 test.
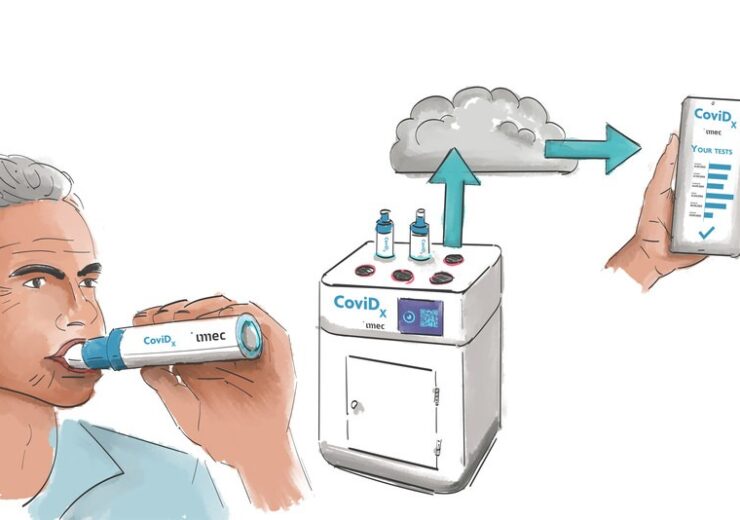
The new test will be designed to identify SARS-CoV-2 virus particles in a person’s exhaled breath, unlike current tests which use blood, saliva, or a nasopharyngeal swab.
The new test will facilitate rapid, easy, and comfortable larger-scale testing, and provides accurate identification in less than five minutes, to help the address the pandemics, said the company.
According to the organisation, polymerase chain reaction (PCR) test is the most sensitive and reliable tool that can detect the genetic material of viruses in a nasopharyngeal specimen.
However, trained medical personnel are needed for collection of the swab, and the process is highly uncomfortable, and requires processing time of about two days.
Rapid antigen tests are alternative to PCR tests, but are less reliable due to complexity. Serological tests are rapid and less expensive than PCR test but detects only whether the person developed antibodies or not.
Imec CEO Luc Van den hove said: “Thanks to a grant of 2 million euro provided by the Flemish government, we are off to a flying start. After all, the speed with which we will be able to bring this test to the market will greatly depend on the proper financial support.
“In anticipation of other investors joining this effort, imec has decided to pre-invest the necessary resources as part of our contribution to the global fight against Covid-19.”
The new test to contain sample collector and analysis unit
The new diagnostic test comprises a sample collector and an analysis unit. The sample collector will serve as the aerosol and virus particle collector, along with supporting the real-time quantitative (RT-q)PCR functionality of the device.
Imec is planning enter into partnership with the UZ Leuven University Hospital for the clinical validation of its new product, and is planning to test a functional prototype at Brussels Airport by the summer of 2021.
After developing the basic technology, experts from the UZ Leuven University Hospital will work together with Imec to participate in a broad clinical study.
Brussels Airport Company CEO Arnaud Feist said: “At Brussels Airport, we are convinced that testing is and will be a key element in the recovery of the aviation sector. Creating a safe environment for our passengers is our top priority and, in this context, it is imperative for us to rely on rapid and reliable testing.”
