I-VASC intends to use the funds to conduct post-market clinical research, gain FDA approval for the US market
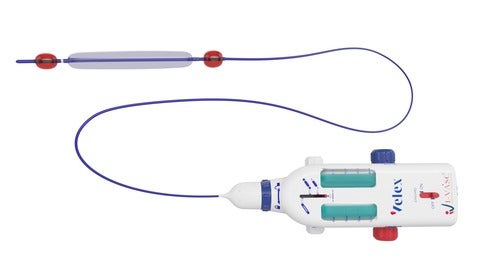
VELEX, a novel device for the treatment of Chronic Venous Insufficiency (CVI). (Credit: Business Wire/ I-VASC Srl)
Italy-based medical device company I-VASC has announced the closing of a €1.8m Series A investment for the launch of its VELEX device.
VELEX, which received CE Mark in May 2021, can be used for the treatment of Chronic Venous Insufficiency (CVI) and varicose veins named Empty Vein Ablation (EVA).
The investment round saw the participation of former shareholders and new investors like Luca Trevisan, Bootes (Rosario Bifulco), and the Nalini family Office.
I-VASC intends to use the funds to conduct post-market clinical research, gain FDA approval for the US market, and complete product industrialisation.
VELEX offers more safety in comparison to conventional schlerotherapy.
I-VASC chief executive officer Daniele Zanotti said: “I am thrilled to embrace this new professional adventure and put my experience at the service of a project which has the potential of representing a new paradigm in the largely underserved market of CVI and varicose vein.
“With the considerable efficacy, safety and usability improvements that VELEX can offer with respect to all alternative methods, we have the opportunity to offer a better option to millions of patients and create a huge new value in the vascular arena.”
The company also appointed two new directors to its board as a part of its strategic growth.
The firm raised €75,000 in the first half of 2021, followed by another €1.066m before the year-end, taking the total amount to €1.8m in 2021.
I-VASC founder and CMO Mario Salerno said: “We are approaching a new and important phase of the project, which I started in 2015.
“Thanks to the investors, who have believed in our proprietary and innovative technology, and to our reinforced team, we are now on the verge to bring VELEX into the market and provide proof of the validity of our EVA (Empty Vein Ablation) procedure.”
