Hardy Diagnostics introduces HealthLink Universal Transport Medium for collection, transport, maintenance and long-term freeze storage of clinical specimens
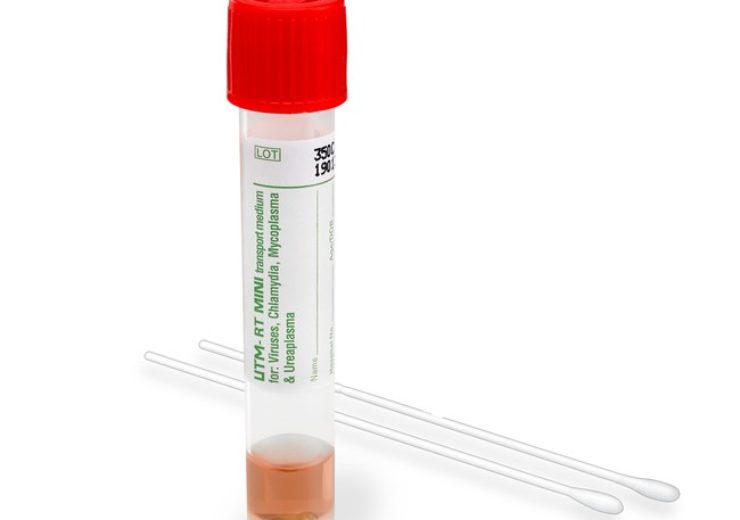
HealthLink Universal Transport Medium UTM by COPAN. Credit: Hardy Diagnostics.
US-based medical devices manufacturer Hardy Diagnostics has unveiled Universal Transport Medium (UTM), an FDA approved collection and transport system for clinical specimens.
The new UTM is intended for collection, transport, maintenance and long-term freeze storage of clinical specimens, comprising viruses, chlamydia, mycoplasma, and ureaplasma organisms.
Hardy vice president and sales and marketing director Christopher Catani said: “We’re incredibly proud to be able to offer the full line of HealthLink UTM products to our customers. COPAN is an excellent partner and we expect high demand for these products because of the aggressive flu season predicted by the CDC.”
UTM is used for rapid antigen testing, DFA, viral culture and molecular-based assays
The UTM, designed as a plastic, screw-cap tube, will maintain the organism viability for 48 hours at room or refrigerated temperature. It is claimed to have been tested and validated in full compliance with quality control of microbiological transport system standard – CLSI M40-A2.
In addition, the unique media formulation of UTM includes antibiotics that inhibit bacterial and fungal growth, without affecting viruses, chlamydia, mycoplasma or ureaplasma.
Hardy Diagnostics is the distributor of the HealthLink UTM, developed and manufactured by Collection and Preservation for Analysis (COPAN), which was founded in 1979, in Mantua, Italy.
COPAN is focused on developing advanced technologies in preanalytics, and its vertically integrated approach enables consistent production of quality products.
In addition, COPAN is said to supply a large number of bacteriology transport swabs, transfer pipets, calibrated plastic inoculation loops, UTM viral transport systems and Flocked Swabs across the world.
Hardy Diagnostics is an FDA-licensed microbiological testing devices manufacturer with an ISO 13485 certified Quality Management System. Headquartered in Santa Maria, California, it services more than 10,000 laboratories across the US.
Established in 1980, the company manufactures more than 2,700 products for the culture and identification of bacteria and fungi, from its manufacturing facilities in California and Ohio in the US.
