Made for diabetic peripheral neuropathy pain, the wearable device monitors pulses of a low-level electrical current over several days
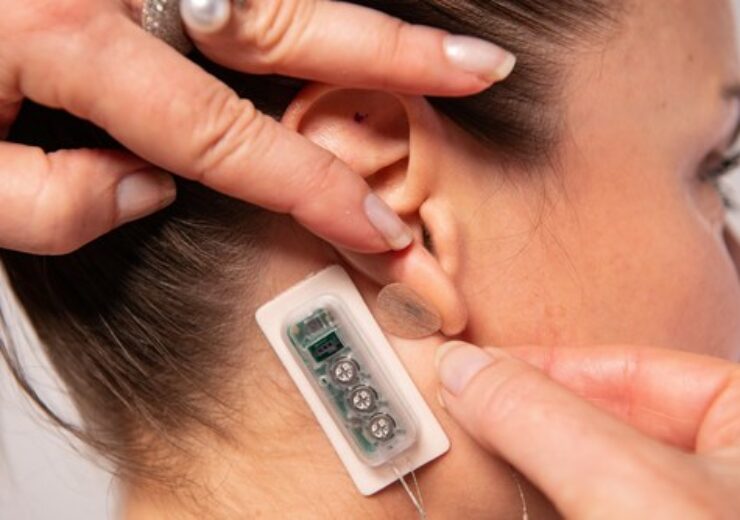
The First Relief is applied behind the ear and delivers continuous pulses of a low-level electrical current over several days. (Credit: PR Newswire/ DyAnsys Inc.)
Diagnostic and monitoring system developer DyAnsys has received approval from the US Food and Drug Administration for its First Relief device.
The First Relief, a PENS (percutaneous electrical neurostimulation) device is intended for multiple treatments for up to 56 days for symptomatic relief of chronic, intractable pain from diabetic peripheral neuropathy.
The wearable device worn on the ear delivers constant pulses of a weak electrical current over several days.
Dyansys said that the FDA’s approval was based on a study comparing First Relief to a placebo and another device that had already received FDA authorisation.
The study was conducted at Jeevak Multispeciality Hospital in Warangal, India, which is well-known for its expertise in treating diabetes.
Dyansys said 63 patients aged between 30 and 74 years participated in the single centre, three-arm, randomised, controlled, parallel assignment, double-blinded, prospective trial.
The First Relief device was administered every two weeks for 16 weeks.
Pain intensity determined by the Visual Analog Scale (VAS) score was the primary efficacy endpoint.
The secondary efficacy endpoints are the vibration perception threshold (VPT) value, insomnia severity index (ISI), overall neuropathy limitations scale (ONLS), and Hamilton rating scale for anxiety.
According to VAS pain score analysis, the pain score of patients being treated with the First Relief device was significantly decreased from the start of the treatment to the end.
The improvement remained over the 90-day follow-up, indicating that the therapy improved neuropathic pain over the long term rather than just temporarily.
The secondary outcome measures also demonstrated changes similar to those in the pain score, demonstrating a notable improvement in mood and sleep as the neuropathic pain reduced.
DyAnsys CEO Srini Nageshwar said: “We are excited to have the FDA clearance of First Relief so that this device, which has been proven effective, can now be used to treat patients who have been experiencing pain related to diabetic neuropathy.
“First Relief offers a significant treatment option without drugs or narcotics.”
