The company plans to commercially launch Flexcera in the US and Canada by the end of June
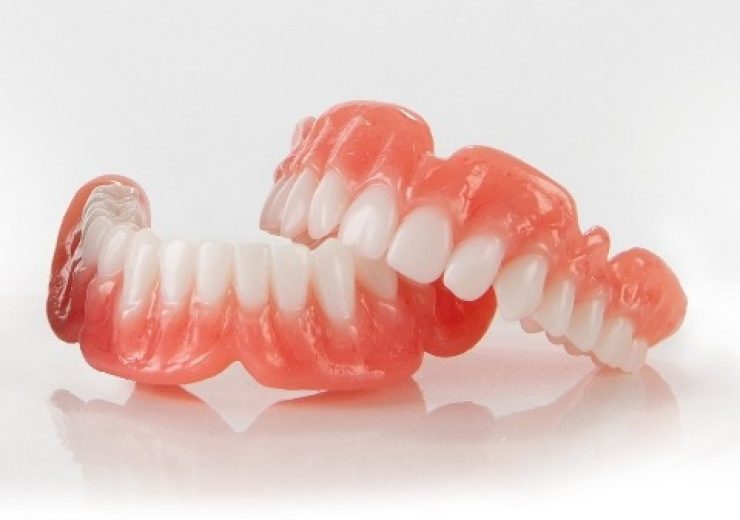
Desktop Health has received FDA approval for Flexcera Base resin for use in 3D fabrication of dental prosthetics. (Credit: Business Wire)
Desktop Health has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its Flexcera Base resin suitable for use in 3D fabrication of dental prosthetics.
Flexcera Base and Flexcera Smile are said to be the company’s first formulated and enhanced digital dental solutions.
Flexcera Smile is an FDA class 1 medical device for the fabrication of lifelike denture teeth, while Flexcera Base is an FDA cleared class 2 medical device for the fabrication of premium denture bases.
Both resins have been formulated exclusively for use with EnvisionTEC 3D printers.
Desktop Health president and CEO Michael Jafar said: “The introduction of Flexcera marks the inception of a remarkable new era in dentistry, combining advanced Flexcera science with 3D printing technology to deliver superior strength, aesthetics, and function for patients.
“We are pleased to bring this innovative product to market as it represents our commitment to meet the needs of dental professionals and their patients.”
The company has developed Flexcera by combining the strength of ceramic with long chain chemistry to maintain optimal denture properties.
The use of EnvisionTEC 3D printers will allow dental providers to print up to eight customised Flexcera dentures within two hours.
Flexcera is claimed to provide high fracture resistance and three times more resistant to fracture than select competitive resins.
According to the company, Flexcera’s moisture resistance enables to avoid staining or discoloration.
Desktop Health, based in Burlington, Massachusetts, plans to commercially launch Flexcera in the US and Canada by the end of June.
