Phase 3 SPOTLIGHT trial evaluates 18F-rhPSMA-7.3, a prostate-specific membrane antigen-targeted radiohybrid (rh) PET imaging agent used in biochemical recurrence of prostate cancer
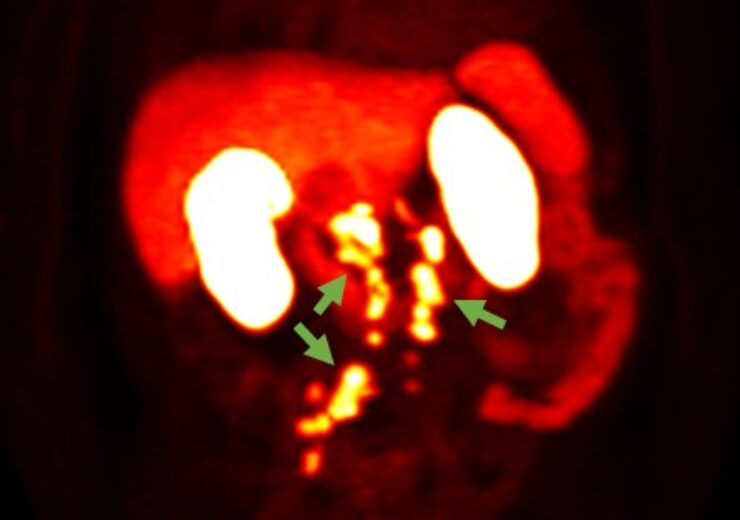
18F-rhPSMA-7.3 PET image showing prostate cancer spread beyond the prostate region. (Credit: Blue Earth Diagnostics)
UK-based PET radiopharmaceuticals developer Blue Earth Diagnostics announced the additional results from its Phase 3 SPOTLIGHT trial of 18F-rhPSMA-7.3 in recurrent prostate cancer.
In a Late-breaking Abstract oral presentation at the 2022 AUA Annual Meeting (AUA2022), researchers reported the impact of 18F-rhPSMA-7.3PET on upstaging patients.
According to the firm, 18F-rhPSMA-7.3 is a radiohybrid (rh) PET imaging drug that targets prostate-specific membrane antigen.
The SPOTLIGHT trial is a multi-centre, single-arm imaging investigation in males with probable prostate cancer recurrence based on high PSA after prior therapy.
The 18F-rhPSMA-7.3 PET data were previously reported at the ASCO GU in February 2022.
Blue Earth Diagnostics, an indirect subsidiary of Bracco Imaging, said that the Correct Detection Rate (CDR) evaluation and its impact on patient upstaging were among the findings presented at AUA2022.
They were based on three blinded, independent PET readers’ individual read outcomes. A composite Standard of Truth was found in the Efficacy Analysis Population (EAP) comprising 366 men.
Blue Earth Diagnostics chief executive officer David Gauden said: “These results from the Phase 3 SPOTLIGHT trial are part of a New Drug Application with the US Food and Drug Administration (FDA) for 18F-rhPSMA-7.3 PET imaging, and we are pleased that they are being presented to the clinical community at the prestigious AUA2022 conference.
“In line with our mission to help patients with cancer, we continue to develop our uniquely comprehensive prostate cancer portfolio, which includes 18F-fluciclovine and investigational rhPSMA compounds for potential use in diagnostic PET imaging and targeted radiopharmaceutical therapy.”
David added: “18F-rhPSMA-7.3 represents a new class of high affinity PSMA-targeted PET radiopharmaceuticals. Early studies of 18F-rhPSMA-7.3 demonstrated high binding affinity for PSMA, together with biodistribution data suggesting the potential for low bladder activity.”
In March 2020, Blue Earth Diagnostics announced the dosing of the first patient in its Phase 3 LIGHTHOUSE clinical trial of targeted PET imaging agent rhPSMA-7.3 (18F) in newly diagnosed prostate cancer.
