BENEFIT-02 is a multicentre, randomised, single-blind, feasibility study that evaluated the effectiveness of Resonance multiphase stimulation used in its Neuro Prospera system for the treatment of patients with chronic pain
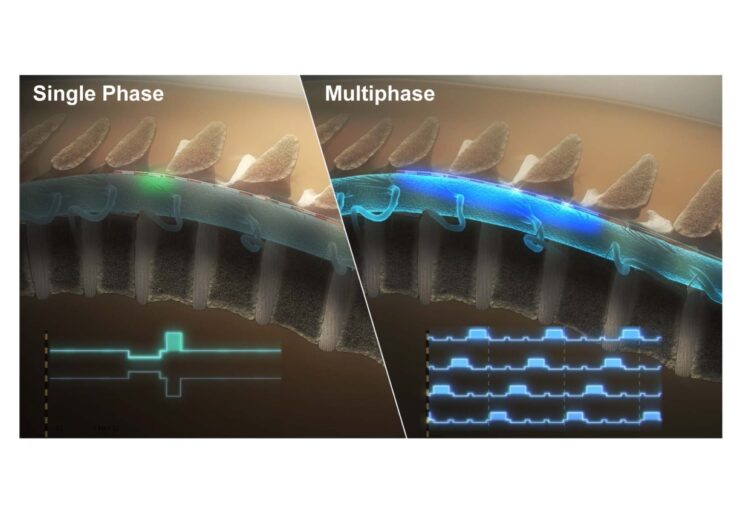
Single phase commercial stimulation vs Multiphase stimulation. (Credit: BIOTRONIK)
German medical device company Biotronik has unveiled positive data for its Neuro Prospera spinal cord stimulation (SCS) system based on results from the BENEFIT-02 trial.
BENEFIT-02 is a multicentre, randomised, single-blind, feasibility study that evaluated the effectiveness of Resonance multiphase stimulation used in its Neuro Prospera system.
The study enrolled 65 participants with chronic pain in the lower back or leg, with a baseline numerical rating scale (NRS) for overall pain intensity of six or above.
All the participants completed the study, with no statistically significant difference in mean NRS reduction or pain relief between multiphase therapies and no serious adverse events.
In the study, 63.9% of participants reported superior pain relief with multiphase than with commercial SCS therapy, along with enhanced average sleep quality and physical activity.
The BENEFIT-02 study, a part of a research programme supporting the Prospera system, was approved by the US Food and Drug Administration (FDA) in March this year.
BENEFIT-02 study principal investigator Leonardo Kapural said: “Former studies have shown good results with previously commercialised SCS technology, yet in practice we are still looking for ways to reduce patient burden and improve outcomes.
“This study is particularly exciting because it’s the first in a series to explore how to do that. The short-term multiphase stimulation results here – combined with early findings from the long-term BENEFIT-03 study – show great promise for the future of SCS.”
Biotronik said that BENEFIT-03 is the first long-term study of its Prospera system with Resonance multiphase stimulation, automatic device monitoring and remote programming.
The interim results from the ongoing study in Australia showed superior pain reduction in both in-clinic and at-home settings with less severe disability.
Resonance requires less power than other SCS therapies and uses an integrated circuit design to deliver a continuous and temporally distributed therapeutic pulse across the spinal cord.
In addition to Resonance, Prospera includes Embrace On, a patient-centric care model that enables automatic, objective, daily remote monitoring, said the medical device company.
Biotronik Neuro president Todd Langevin said: “As we await additional data from our BENEFIT-03 study, we’re excited to see these initial data on the long-term effects of our SCS system.
“We developed Prospera to offer patients sustainable pain relief – and we believe its proactive care model will have a clinically meaningful impact on lowering long-term failure rates and reducing the service burden of SCS.”
