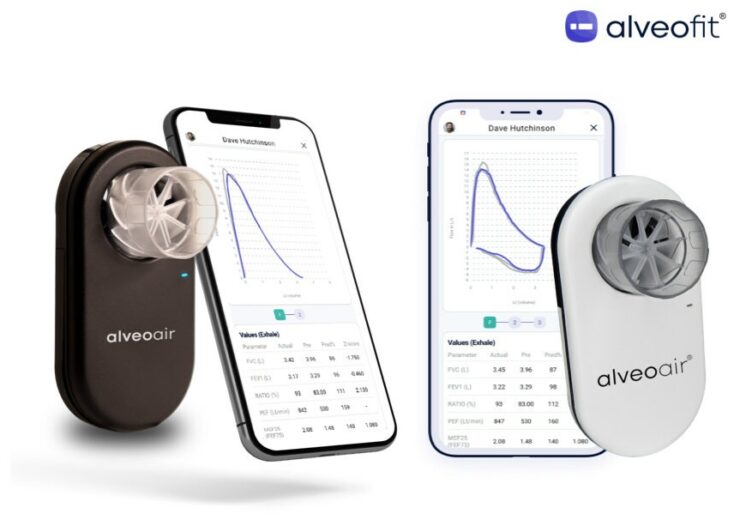
Alveoair is a personal spirometer that leverages the company’s advanced accu-sense technology to help patients with asthma, COPD, cystic fibrosis and ILD to quantify their lung health and offers real-time insights to healthcare professionals
The alveofit Ecosystem. (Credit: PRNewswire/ alveofit)
India-based Roundworks Technologies (alveofit) has received the US Food and Drug Administration (FDA) approval for its portable digital spirometer product, alveoair.
Alveoair is a personal spirometer designed to help patients with asthma, COPD, cystic fibrosis and ILD, to measure their lung health.
The alveoair platform leverages the company’s advanced accu-sense technology and offers real-time insights to healthcare professionals, enabling timely interventions in respiratory care.
The insights, in conjunction with AI models, support predictive analytics into respiratory health, which enables clinicians to prioritise cases and deliver quality care.
India’s Central Drugs Standard Control Organisation (CDSCO) granted regulatory certification to the alveoair spirometer in June last year.
Since then, the device has been adopted by more than 400 healthcare facilities across India.
Alveofit said that the FDA approval, together with strategic partnerships, will support the company’s plans for international expansion, specifically in the US and emerging economies.
The company collaborates with academic institutions, such as AIIMS Delhi, for clinical research, and has partnered with Tatvacare to extend digital respiratory care solutions to patients’ homes.
It also collaborates with companies like AstraZeneca, organisations like India Sweden Innovation Centre (ISIHC), NASSCOM COE, PATH, Forge Innovation & Ventures, and DST.
NASSCOM COE CEO Sanjeev Malhotra said: “As alveofit ecosystem is scaled PAN India, It is good to see the product mature over the last couple of years and gain acceptance among the providers.
“I congratulate and wish alveofit a global success with recent approval from FDA, making a difference in the lives of people in India and world over.”
PATH South Asia Director and India country director Neeraj Jain said: “PATH is actively supporting their spirometry project in Maharashtra’s Satara district for early diagnosis of chronic respiratory conditions.”
