The next generation Hemolung RAS is based on intellectual property licenced from the University of Pittsburgh
ALung has commenced the commercial development of next-generation artificial lung. (Credit: ALung Technologies, Inc)
ALung Technologies, a provider of advanced lung assist devices, has commenced the commercial development of the next-generation artificial lung.
The company’s Hemolung respiratory assist system (RAS) is claimed to be the only fully comprehensive extracorporeal carbon dioxide removal (ECCO2R) system developed for this therapy.
The next generation Hemolung RAS is based intellectual property licenced from the University of Pittsburgh.
The new technology platform is developed by professor William Federspiel and his colleagues at the Swanson School of Engineering and the McGowan Institute for Regenerative Medicine.
The platform enables to significantly improve gas exchange efficiency and minimise the deleterious hematologic effects from extracorporeal blood circulation.
ALung has CE mark approval for Hemolung RAS
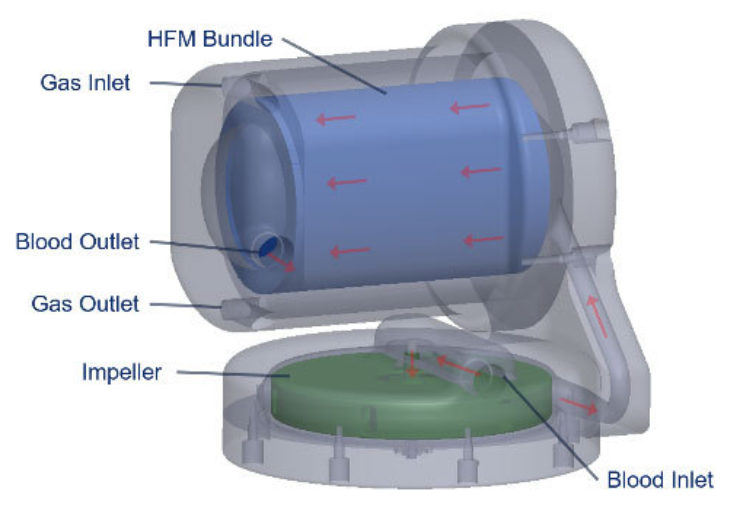
The next-generation Hemolung RAS will feature patent-pending technology that helps to create better blood flow uniformity to enhance gas exchange efficiency.
It will also include a custom-designed centrifugal pump incorporated with a low surface area (0.65 m2) gas exchange membrane and avoids the use of additional components to minimise operational complexity of the system.
The next-generation system provides low flow ECCO2R (250 – 700 mL/min) and full ECMO (2 – 4 L/min) by using a single integrated pump and gas exchange membrane.
At present, the company has CE mark approval for Hemolung RAS. It has also been assessed for safety and efficacy in two large pivotal trials such as the FDA-approved VENT-AVOID trial and the UK REST trial.
ALung Technologiesc chairman and CEO Peter DeComo said: “The next generation Hemolung RAS is a direct result of the continued collaboration between the University of Pittsburgh and ALung Technologies.
“This collaboration, spanning 20+ years, has resulted in a rich pipeline of innovation for ALung that will accelerate the development of highly efficient, simple to use artificial lung devices for the treatment of acute respiratory failure.”
Recently, ALung Technologies secured emergency use authorisation (EUA) from the US FDA for the Hemolung RAS to treat patients with Covid-19.
