Olympus announced an expansion of coverage for a treatment that has been demonstrated to improve quality of life for eligible patients suffering from severe emphysema, a form of COPD (Chronic Obstructive Pulmonary Disease).
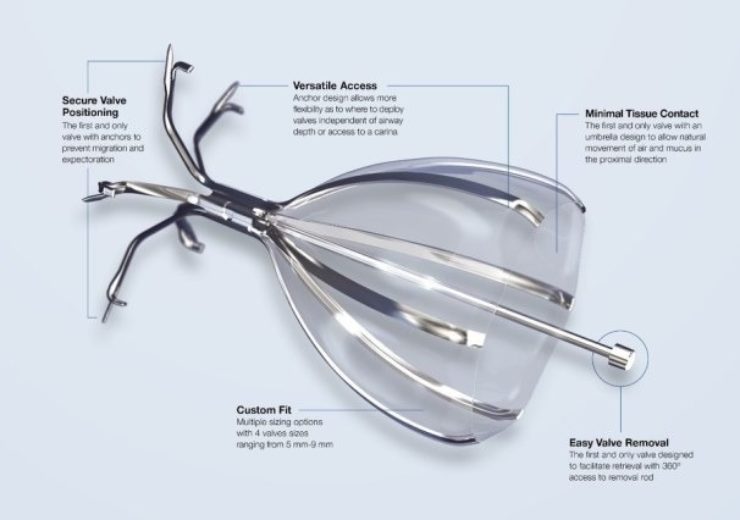
Image: The Spiration Valve is an umbrella-shaped device made up of five anchors that securely engage the airway walls at targeted treatment locations. Photo: courtesy of PRNewswire / Olympus Medical Systems Group.
The nation’s largest commercial insurance company, UnitedHealthcare, now provides coverage for eligible patients treated with endobronchial valves for bronchoscopic lung volume reduction (BLVR). Serving nearly 50 million medical members worldwidei, UnitedHealthcare now joins other payers in the U.S. covering this minimally invasive treatment for people suffering from severe emphysema.
Endobronchial valves used for BLVR, including the Spiration Valve System (SVS), are removable devices placed into selected airways of emphysematous lung during a short bronchoscopic procedure. Once in place, the valves redirect air from diseased parts of the lung, allowing healthier lung tissue to expand and function more effectively. Reducing lung volume has been shown to allow patients to breathe more easily and experience improvement in their quality of life.ii
“We are gratified that, through UnitedHealthcare’s expanded coverage, more patients will have access to this important therapeutic treatment for relief of symptoms from severe emphysema,” said Lynn Ray, Olympus’ Global General Manager and Vice President for Respiratory. “This is a notable signal, among others, that endobronchial valve treatment such as the SVS is an option for treating eligible patients.”
BLVR Procedure Using Endobronchial Valves Upgraded to Highest GOLD Rating
The 2020 report from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) upgraded the evidence level rating for BLVR with endobronchial valves to its highest “A” rating. According to the report, this evidence rating is based on results from randomized clinical trials with data from more than two clinical trials involving a substantial number of patients, including those treated with the SVS.iii
“The GOLD rating confirms that the body of evidence supporting endobronchial valves is significant and supports the use of this intervention as standard of care for patients suffering from severe emphysema, a form of COPD,” said Dr. Gerard Criner, Professor of Medicine and Chair of the Department of Thoracic Surgery and Medicine at Temple University. “Before endobronchial valves such as the Spiration Valve were FDA approved, the only option for patients on maximum medical management was major surgery, which comes with significant risks. With significant evidence now in place for endobronchial valve treatment, we can now offer patients a choice in treatment options.”
GOLD’s Global Strategy for Diagnosis, Management and Prevention of COPD report is used by healthcare professionals worldwide in establishing their strategies for managing and preventing COPD, a disease that impacts more than 65 million people globally. GOLD first added mention of the benefits of bronchoscopic interventions for COPD, including endobronchial valve treatment like SVS, in 2017. The report is reviewed and revised annually by leading physicians in the field of COPD.
Olympus is a global technology leader, crafting innovative optical and digital solutions in medical technologies; life sciences; industrial solutions; and cameras and audio products. Throughout our 100-year history, Olympus has focused on being true to society and making people’s lives healthier, safer and more fulfilling.
Source: Company Press Release
