The agreement allows Antisoma Therapeutics to gain exclusive, worldwide access to the sensor technology in the fields of respiratory viral disease
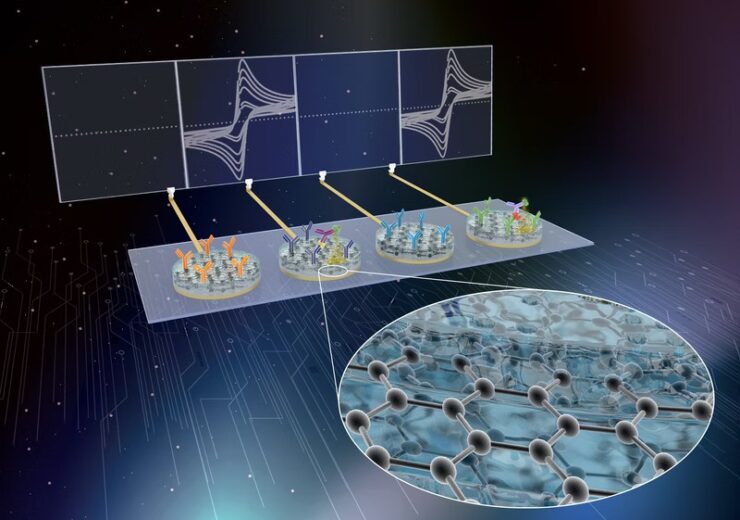
The technology is licenced to enable the development of new diagnostics for respiratory viral diseases. (Credit: Wyss Institute at Harvard University)
Wyss Institute for Biologically Inspired Engineering at Harvard University has licenced the electrochemical eRapid technology to Antisoma Therapeutics.
Under the licensing agreement, Antisoma Therapeutics will gain exclusive, worldwide access to the sensor technology in the fields of respiratory viral disease, including COVID-19 and the flu, allergic responses and anaphylaxis, and cancer.
The agreement allows the subsidiary of Australian biotech company The iQ Group Global to jointly develop point-of-care diagnostics.
In line with its commitment to the COVID-19 Technology Access Framework, Harvard has initially provided access to the eRapid technology for The iQ Group Global on a non-exclusive basis, for a limited term.
Wyss Institute founding director Donald Ingber said: “After we realized in 2019 the enormous future potential of eRapid for monitoring various disease pathologies, we systematically de-risked the technology and successively added and validated the features that enabled it to mature into the powerful diagnostic platform it is today.
“This license marks an important moment in moving eRapid technology out into the world where it could improve the lives of patients suffering from infectious, immune, or cancer diseases, whose rapid diagnosis requires the detection of multiple biomarkers.”
eRapid has been developed as a low-cost, affinity-based electrochemical sensing platform with a capability to simultaneously detect and quantify a range of biomarkers in a small volume of blood and other complex biological fluids.
The technology allows the attachment of ligands for a range of biomarkers, including RNAs, proteins, host antibodies, and metabolites, to a novel antifouling nanocomposite coating.
Later, the eRapid platform produces an electrical signal within minutes after chemically detecting a target biomarker.
The iQ Group Global CEO and chairman George Syrmalis said: “Our priority is to continue developing and commercializing the saliva quantitative COVID test, standardised against the World Health Organization reference preparation, which will allow patients and physicians to determine immunity based on antibody load, and thus determine if and when additional vaccination shots are needed.
“This level of accuracy will rationalise repeat vaccination protocols, increase the effiency of health care services while dramatically reducing costs to governments and the health care sector.”
