The device was designed to address unmet needs in complex hernia repair, providing rapid vascularity3 and permanent strength with a low profile for single, effective hernia repair.
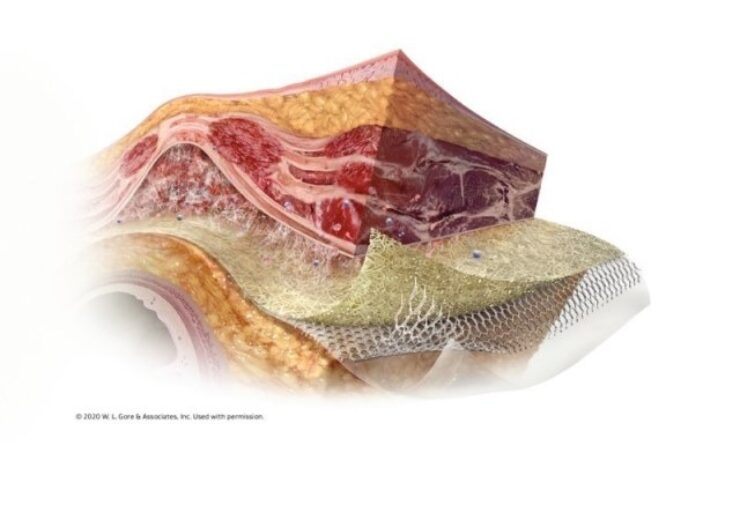
GORE SYNECOR intraperitoneal biomaterial offers a tri-layer hybrid solution that supports single, effective hernia repair in complex patients. (Credit: PRNewswire / W. L. Gore & Associates, Inc. MPD Division)
W. L. Gore & Associates (Gore) announced that GORE SYNECOR Intraperitoneal Biomaterial is now available in Europe, Middle East and South Africa.
The device was designed to address unmet needs in complex hernia repair, providing rapid vascularity3 and permanent strength with a low profile for single, effective hernia repair.
GORE SYNECOR Intraperitoneal Biomaterial is designed for ease of use during laparoscopic, robotic and open surgical procedures. The tri-layer hybrid material was first approved and launched in 2016 in the United States and received CE
“This global expansion introduces a new category of synthetic hybrid material in Europe, Middle East and South Africa for surgeons to use in complex ventral hernia repair,” said David Lane, General Medical Products Business Leader at Gore.
Bringing the latest innovations to hernia repair and abdominal wall reconstruction
As pioneers in the mid-term bioabsorbable mesh category, Gore is persistent in the innovation of material solutions so that surgeons have quality material options when assessing the risk for complications in complex cases. GORE SYNECOR Intraperitoneal Biomaterial is flexible and conformable, with memory for easy unrolling, handling and optimal placement. The material absorbs bodily fluids and no pre-soaking is needed.
The unique three layers of GORE SYNECOR Intraperitoneal Biomaterial are designed to promote rapid vascularity with minimal permanent material3 (data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ).
Parietal Surface: Gore 3D PGA:TMC web scaffold generates high-quality tissue via rapid cellular infiltration. Vascularization is reported within seven days3 and tissue ingrowth within one month (data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ).
Mid-Layer: Macroporous knit of dense monofilament PTFE fibers designed with a fiber diameter similar to lightweight mesh but with the strength of heavyweight mesh.
Visceral surface: Non-porous PGA:TMC film provides intra–abdominal protection, minimizing the risk of adhesion formation to the material4 (data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ).
To learn more, register to attend the Gore Complex Ventral Hernia Symposium on March 16, 2021.
“I am very happy to see a new prosthetic material, with excellent clinical background and promising added-value in tissue integration under the new paradigm in minimally invasive abdominal wall reconstruction,” said Dr. Salvador Morales-Conde, General Digestive Surgeon, Chief of Innovation Unit, Sevilla, Spain.
GORE SYNECOR Intraperitoneal Biomaterial is part of a portfolio of surgical devices featuring Gore’s proven synthetic material, including GORE® BIO-A® Tissue Reinforcement, GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement, GORE-TEX® Soft Tissue Patch and GORE® DUALMESH® Biomaterial.
Source: Company Press Release
