In this multi-center, randomized, double-blind, placebo-controlled trial, the primary endpoint is to investigate whether unfocused electro-hydraulic shockwaves (uESWT aka SoftWaves) can achieve a statistically significant improvement in motor and sensory function (the AIS grade) for acute spinal cord injuries
Softwave Tissue Regeneration Technologies announces landmark trial for acute traumatic spinal cord injuries. (Credit: Softwave Tissue Regeneration Technologies.)
SoftWave Tissue Regeneration Technologies, a disruptive bio-medical device company, announces the beginning of a trial for “Extracorporeal Shock Wave Therapy in Acute Traumatic Complete and Incomplete Cross – Sectional Lesions.” The trial will be sponsored by the Austrian Workers’ Compensation Board (AUVA). It will be conducted in 13 hospitals in Austria and one in Germany, over the next two years.
In this multi-center, randomized, double-blind, placebo-controlled trial, the primary endpoint is to investigate whether unfocused electro-hydraulic shockwaves (uESWT aka SoftWaves) can achieve a statistically significant improvement in motor and sensory function (the AIS grade) for acute spinal cord injuries, if applied within the first 72 hours of said injury. Assessments on the ability to walk, quality of life, pain, and everyday independence will be compared over time in each group.
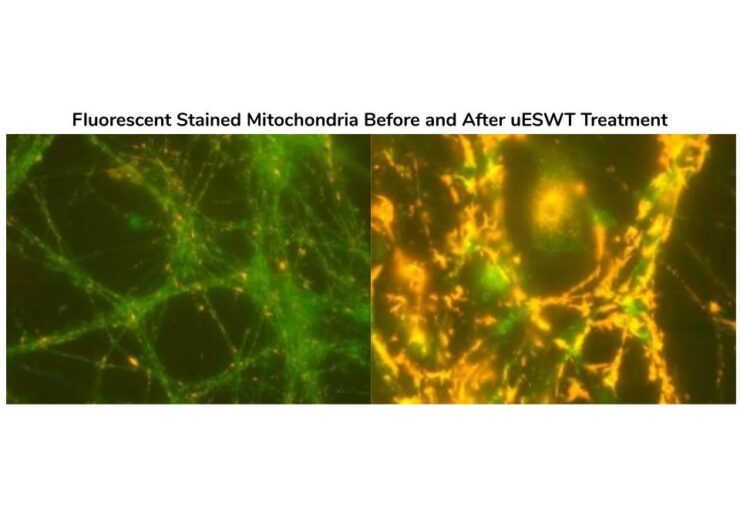
The hypothesized mechanisms of action associated with the improved clinical outcomes include the following: stem cell activation, a reduction in apoptosis, neovascularization, and, most importantly, a reduction in the inflammatory response post-injury. The aforementioned reduction is due to SoftWave’s known ability to regulate inflammation via the Toll Like Receptor 3 pathway (TLR3).
Medical research shows that swelling after spine trauma may disrupt the remaining viable spinal cord function, even after the injury has been stabilized. Swelling is the enemy of SCI patients in the early hours and days post-trauma. SoftWaves dramatically reduce swelling, offering hope to SCI patients, if applied early after an injury. Previous in vitro and animal trials suggest that SoftWaves may reduce the degree of paralysis, if applied within hours of an injury. The treatment dramatically reduces the inflammation and initiates a strong biologic cascade, including stem cell activation and neovascularization.
Source: Company Press Release
