Regeneten is a collagen-based implant, designed to support the body’s natural healing response to facilitate the growth of new tendon-like tissue which will biologically extend into the existing tendon and change the course of disease progression
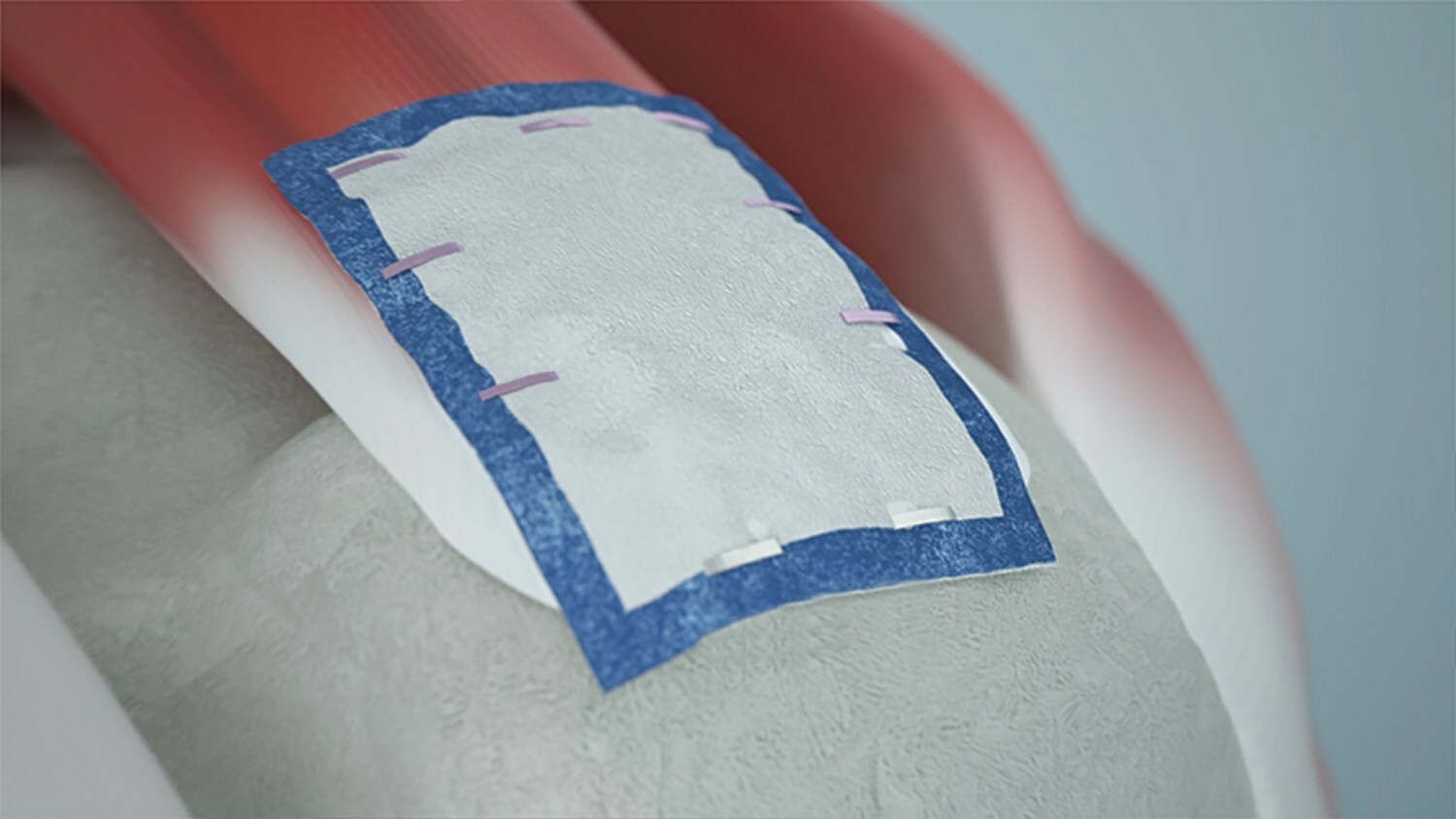
Smith+Nephew’s Regeneten Bioinductive Implant. (Credit: PRNewswire/Smith & Nephew plc)
UK-based medical equipment manufacturer Smith+Nephew has introduced its Regeneten Bioinductive Implant in India, to treat rotator cuff disease.
Regeneten is a collagen-based implant, designed to support the body’s natural healing response to facilitate the growth of new tendon-like tissue.
The newly grown tendon-like tissue will biologically extend into the existing tendon and change the course of disease progression.
Designed to be about the size of a postage stamp, Regeneten implant is delivered arthroscopically through a small incision over the location of the rotator cuff tendon injury.
Within six months from the delivery, the implant will disappear and induce the growth of new tendon-like tissue and orientation of collagen suggestive of functional loading.
Smith+Nephew South Asia managing director Joaquin Lasso said: “We are beyond excited to bring the REGENETEN Bioinductive Implant to India and advance the standard of care for patients suffering from rotator cuff disease.
“The clinical evidence speaks for itself – REGENETEN Bioinductive Implant is changing the game in rotator cuff repair and generating positive outcomes for thousands of patients all over the world.”
According to the company, Regeneten is part of its comprehensive portfolio of advanced healing solutions and supports biological healing in rotator cuff repair.
The bioinductive implant has already completed more than 100,000 procedures and had transformed the way surgeons approach rotator cuff procedures.
Smith+Nephew said that the launch of Regeneten Bioinductive Implant in India builds on more than 10 years of positive results in 18 clinical studies, covering the entire spectrum of rotator cuff disease, from partial to large thickness tears, said the company.
Recent data from a triple-blinded showed that the Regeneten Bioinductive Implant delivered an 86% relative reduction in re-tear rates, compared to conventional surgery alone.
Last month, Smith+Nephew received the US Food and Drug Administration (FDA) 510(k) approval for the AETOS shoulder system for both anatomic and reverse total shoulder arthroplasty.
