SetPoint intends to use the proceeds to support a trial of its bioelectronic platform to treat rheumatoid arthritis
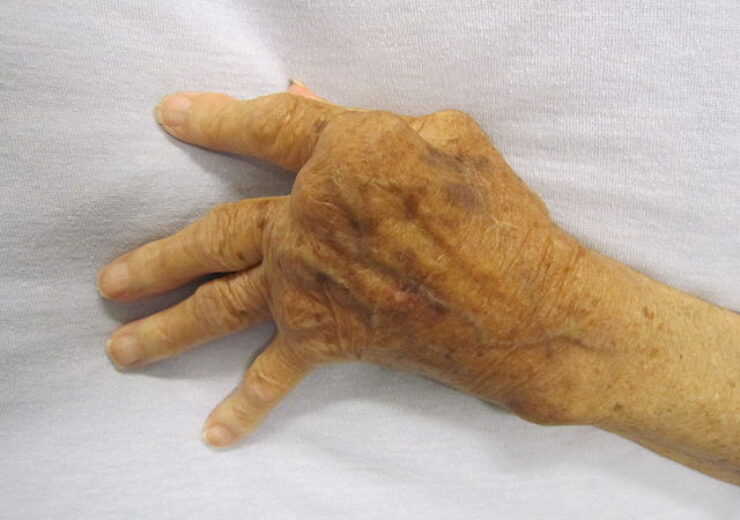
A hand severely affected by rheumatoid arthritis. (Credit: James Heilman, MD)
SetPoint Medical has secured $64m in preferred stock financing to support the pivotal trial of its novel bioelectronic platform.
With an initial focus on rheumatoid arthritis (RA) disease, the firm is developing its platform to treat chronic and inflammation-mediated autoimmune diseases.
The financing round, which was led by New Enterprise Associates (NEA), included returning investors such as Action Potential Venture Capital, Boston Scientific, Topspin Fund, Morgenthaler, Euclidean Capital and an undisclosed strategic investor.
New investors consist of ShangBay Capital, Richard King Mellon Foundation, Ascendum Capital, Asahi Kasei, Catalio Capital Management, BPC Fund, and Midas Capital, among others.
With the financing, ShangBay Capital’s founding managing partner William Dai has joined SetPoint Medical’s board of directors.
SetPoint will use the proceeds from the financing to support its RESET-RA multicentre, double-blind, randomised and sham controlled pivotal trial, which will recruit up to 250 patients at 40 clinical trial sites across the US.
The study will assess the safety and effectiveness of the SetPoint bioelectronic platform in patients with moderate-to-severe RA who are incomplete responders or are intolerant to biologic or targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs).
SetPoint’s bioelectronics device has FDA breakthrough designation
SetPoint already secured breakthrough designation from the US Food and Drug Administration (FDA) for the device to treat patients with RA who have incomplete response to or are intolerant to multiple biologic drugs.
SetPoint Medical president and CEO Murthy Simhambhatla said: “We’re incredibly pleased to have the support of an experienced and strong group of investors who see the disruptive potential of our therapeutic platform.
“We look forward to initiating our pivotal trial in RA and working collaboratively with the FDA to bring our novel therapeutic platform to patients.”
In June 2019, SetPoint Medical first announced positive results from the US pilot investigational device exemption (IDE) study evaluating its bioelectronic medicine device in rheumatoid arthritis.
