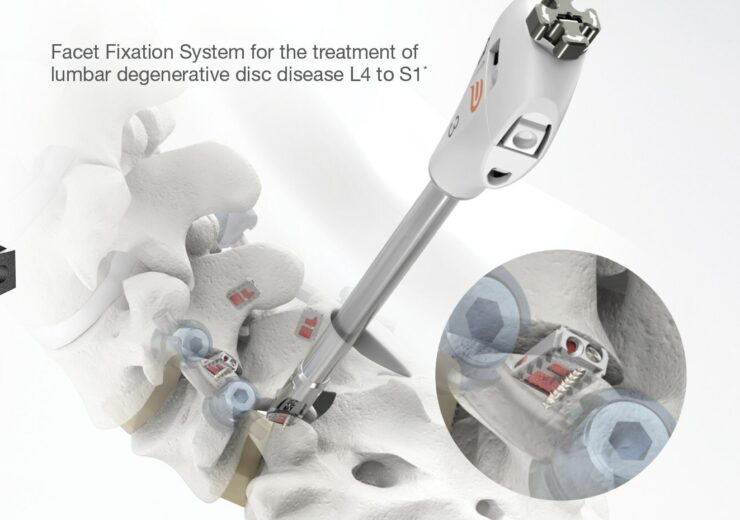
The Cavux FFS-LX is an integrated cage and screw system designed to be implanted bilaterally in the facet joints to treat lumbar degenerative disc disease (DDD) and can also be used with or without pedicle screws and rods
Cavux FFS-LX Lumbar Facet Fixation System. (Credit: PRNewswire/ Providence Medical Technology, Inc.)
Providence Medical Technology (PMT) has received the US Food and Drug Administration (FDA) approval for its lumbar facet fixation system, Cavux FFS-LX, for lumbar spinal fusion surgery.
Cavux FFS-LX is an integrated cage and screw system designed to be implanted bilaterally in the facet joints to treat lumbar degenerative disc disease (DDD).
The implant spans the facet interspace with points-of-fixation at each end of the construct to offer additional stabilisation for 1- or 2-level lumbar interbody fusion.
Cavux FFS-LX can also be used with or without pedicle screws and rods, implanted using the company’s Corus Spinal System-LX tissue-sparing access and spinal fusion system.
Providence Medical Technology co-founder and CEO Jeff Smith said: “At Providence, we are driven to improve clinical outcomes and prevent surgical failures for high-risk patients.
“Cavux FFS-LX builds off our successful cervical platform and applies the same principles to the lumbar spine where the challenge of treating high-risk patients is even more pronounced.
“We are excited to be launching into the lumbar market and provide high-risk patients a new option for a fusion success.”
PMT said that the FDA approval marks its expansion into the $2bn lumbar spine market, which is characterised by a higher surgical failure rate than in the cervical spine.
Patients who fail to fuse after a lumbar spine fusion surgery face a substantial risk of added complications, suffering, and costly revision procedures.
Cavux FFS-LX has been designed to offer enhanced stabilisation after lumbar fusion procedures to increase fusion rates and reduce future complications and reoperations.
The FDA approval was based on data from a clinical study of the Cavux FFS-LX lumbar facet fixation system in 57 patients, which showed a strong safety and efficacy profile.
In the study, 79% of subjects achieved clinically meaningful improvement in pain.
Washington Spine and Scoliosis Institute founder, orthopaedic spine surgeon Joseph O’Brien said: “I am excited about the FDA clearance of Cavux FFS-LX as a treatment option for patients treated with lumbar fusion for degenerative disc disease.
“The implant provides increased stabilisation at the lumbar facet level for minimally invasive fusion procedures.
“This new option will expand the spine specialist’s treatment arsenal and likely lead to increased fusion rates for patients. I have seen excellent results from this technology in challenging fusion patients.”
