As per Orthofix Medical, the M6-C IDE study was a prospective, non-randomized, concurrently controlled clinical trial, which was conducted across 23 sites in the US, with an average patient age of 44 years. In the study, safety and effectiveness of the M6-C artificial cervical disc was compared to ACDF for the treatment of single level symptomatic cervical radiculopathy with or without cord compression.
The overall rate of success for the protocol-specified primary endpoint for the M6-C disc patients was 86.8% at 24 months and 79.3% in the control group.
Orthofix Medical claims that the patients treated with the M6-C artificial cervical disc showed significant improvements in neck, arm pain, function and quality of life score. Additionally, the patient also showed significant difference in the reduction of pain and opioid medications use when compared to anterior cervical discectomy and fusion (ACDF) patients.
Orthofix Spine global president Brad Niemann said: “With the recent FDA approval of the M6-C artificial cervical disc, we are excited to be able to provide spine surgeons and their patients a new and innovative alternative to the ball-and-socket artificial disc designs currently available in the U.S.
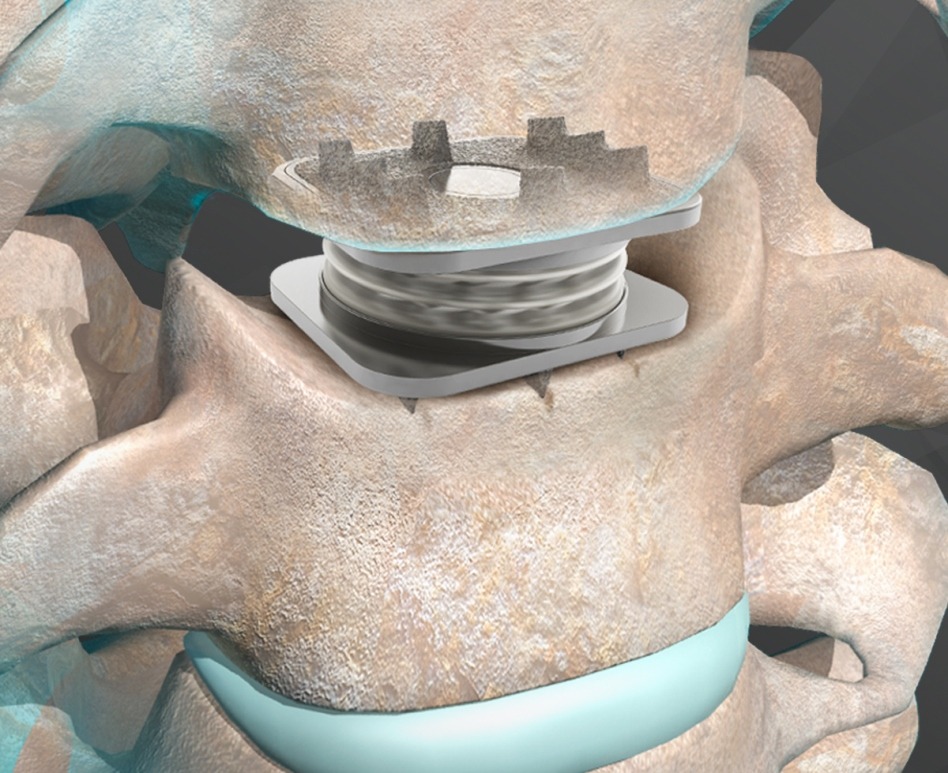
“What makes the M6-C disc so unique is its physiologic single-piece design featuring an artificial nucleus and annulus that work together to mimic the biomechanical motion of a natural disc to include axial compression or shock absorption, which no other disc available in the U.S. has to offer.”
In pain scores, 91.2% of patients who received M6-C disc reported improvement in neck pain compared to 77.9% in patients who had undergone ACDF procedure.
90.5% of M6-C patients also reported improvement in arm pain compared to 79.9% of ACDF patients.
Before undergoing the surgery, 80.6% of the M6-C disc patients and 85.7% of the ACDF patients were taking some kind of pain medication for treating their cervical spine condition.
At 24 months, the rate of M6-C patients who were still taking some type of pain medication dropped to 14.0% compared to 38.2% of the ACDF patients.