Developed specifically for use with the JuniOrtho Plating System, the OrthoNext digital platform software enables the surgeon to accurately plan the osteotomy position to visualize the implant in relation to the anatomy
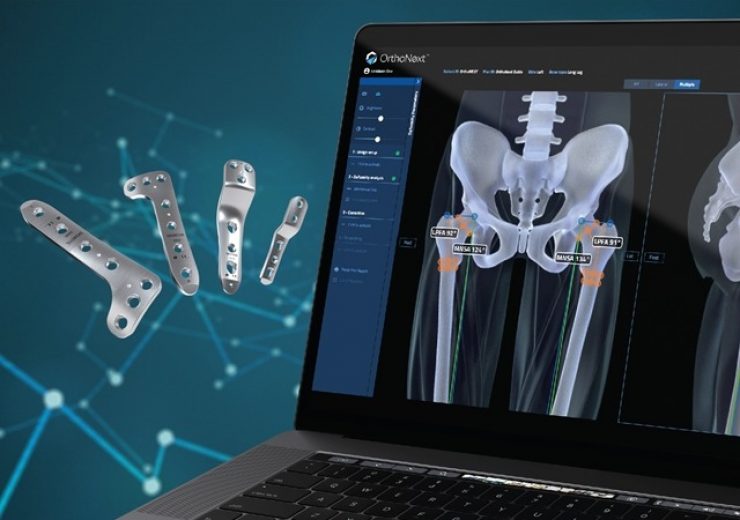
The OrthoNext digital platform enables deformity analysis and preoperative planning for pediatric orthopedic procedures with the JuniOrtho Plating System. (Credit: Business Wire)
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced expansion of its pediatric offerings with the U.S. Food and Drug Administration (FDA) 510(k) clearance of the OrthoNext™ digital platform, the only software tool in the market for deformity analysis and preoperative planning for pediatric orthopedic procedures.
Developed specifically for use with the JuniOrtho Plating System™, the OrthoNext digital platform software enables the surgeon to accurately plan the osteotomy position to visualize the implant in relation to the anatomy. This unique platform is designed to streamline the selection of the precise size of device and enable optimal positioning for the patient’s body prior to the surgical procedure.
With this clearance, Orthofix has also initiated its U.S. and European full market launch of the JuniOrtho Plating System. Created specifically for pediatric patients, the JuniOrtho Plating System is designed to address the demands of advanced deformity and trauma reconstruction of the lower extremities.
“Together, OrthoNext and the JuniOrtho Plating System provide a complete solution for surgeons looking for a plating system to address the specific demands of advanced deformity and trauma reconstruction of the lower extremities in the pediatric population,” said Orthofix President of Global Orthopedics Paul Gonsalves. “We are very pleased to receive the FDA clearance of the OrthoNext platform and excited to move forward with our full market launch of the JuniOrtho Plating System as we continue to deliver on our commitment to advancing pediatric orthopedics.”
The JuniOrtho Plating System is offered in a wide range of plate sizes with a variety of lengths and accommodates both locking and non-locking screws corresponding to the plate size. The system is comprised of sterile implants and single-use tools to optimize efficiency during the procedure.
The OrthoNext platform and the JuniOrtho Plating System will be on display at the Pediatric Orthopaedic Society of North America (POSNA) annual meeting in Dallas, Texas May 12-15. For those attending POSNA, please visit booth 13 to learn more about Orthofix’s JuniOrtho line of pediatric solutions that includes the TL-HEX TrueLok Hexapod System™, TrueLok™ Ring Fixation System, eight-Plate Guided Growth System+, and many others. JuniOrtho brings products and resources together to give both medical professionals and families the best in pediatric orthopedic solutions.
Source: Company Press Release
