Nexpowder endoscopic haemostasis system comes with a unique delivery system that clears the clogged catheters and cloudy fields of vision, to provide gastroenterologists with improved visibility and control of upper gastrointestinal (GI) bleeding
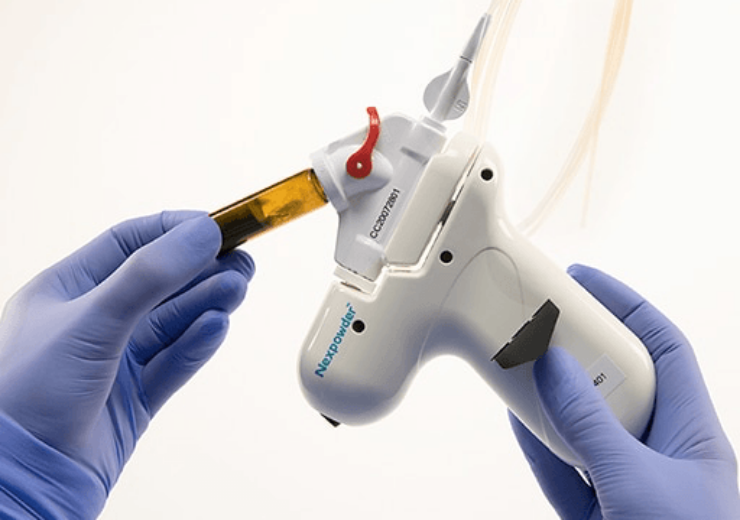
Nexpowder endoscopic haemostasis system. (Credit: Medtronic)
Healthcare technology company Medtronic has received the US Food and Drug Administration (FDA) approval for its Nexpowder endoscopic haemostasis system.
Nexpowder system is designed to provide gastroenterologists with improved visibility and control of upper gastrointestinal (GI) bleeding.
It has a distinctive delivery system that clears the clogged catheters and cloudy fields of vision, without needing CO2 or air compressors.
The system leverages a non-contact, non-thermal and non-traumatic haemostatic powder, sprayed onto a target site endoscopically through a catheter.
Medtronic Gastrointestinal business president Gio Di Napoli said: “We are very excited to bring the innovative, Nexpowder system to gastroenterologists.
“We considered the potential impact on physicians and patients alike, by meeting a clear need to reduce mortality from upper GI bleeding, a condition that causes death for one out of every 1,000 people while also reducing rebleeding, which happens in 20% of all upper GI bleeding cases.
“The Medtronic GI business is striving – across all products in our portfolio – to create transformative technologies that improve outcomes and change the standard of care.”
The catheter, which connects to a spray handle, has a unique hydrophilic powder coating that minimises clogging and improves visibility and control over upper GI bleeding.
After spraying, Nexpowder will immediately form a mucoadhesive and durable gel upon contact, regardless of blood, which degrades in one-to-three days.
The spray attaches the tissue with double the adhesive force of other commercially available products, providing a long-lasting haemostatic effect.
Nexpowder offers a precise solution for nonvariceal upper GI bleeding with minimal clogging, which facilitates direct endoscopic visibility without impairment, said the company.
Medtronic Gastrointestinal business chief medical officer Austin Chiang said: “With a 94% immediate haemostasis rate and a 3.7% rebleeding rate, we’re thrilled to share this effective technology with gastroenterologists.
“When we treat patients, we’re looking for immediate and lasting results when it matters most. The Nexpowder system is a powerful tool for GI professionals to add to their toolboxes.”
