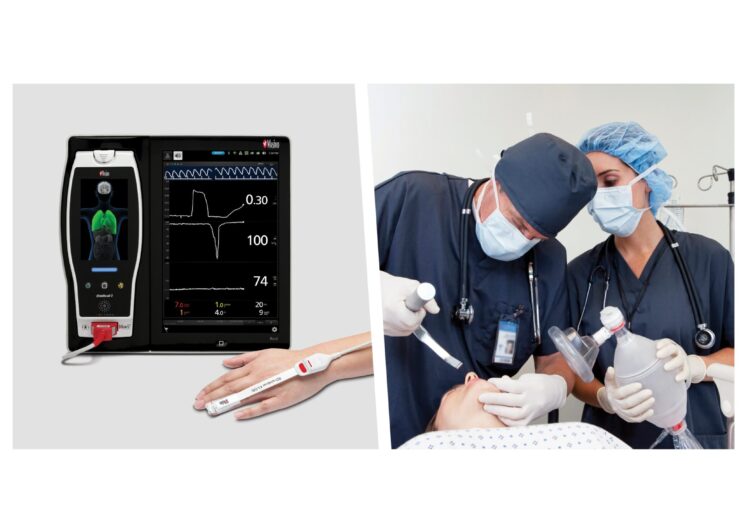
ORi is intended to be used in conjunction with oxygen saturation (SpO2) to provide increased resolution of changes in oxygenation under supplemental oxygen
An image of Masimo ORi. (Credit: Business Wire)
US-based medical technology company Masimo has received De Novo authorisation from the US Food and Drug Administration (FDA) for its noninvasive, continuous parameter, ORi.
The company has designed ORi to provide additional insights into a patient’s oxygen status in the moderate hyperoxic range under supplemental oxygen.
ORi is intended to be used in conjunction with oxygen saturation (SpO2) to provide increased resolution of changes in oxygenation under supplemental oxygen. It is enabled by the multi-wavelength Masimo rainbow Pulse CO-Oximetry technology.
With the De Novo, ORi is said to become the first parameter of its kind approved by the FDA to assist doctors in managing the oxygen of adults having surgery in perioperative hospital settings.
According to the medical technology firm, the continuous parameter offers continuous insight into the oxygenation of haemoglobin in the moderate hyperoxic range.
To increase patient visibility beyond SpO2 under supplementary oxygen, ORi is trended continuously with SpO2 as a unit-less index between 0.00 and 1.00.
In addition to Masimo SET pulse oximetry, ORi gives clinicians more insight into when oxygenation increases or decreases out of moderate hyperoxia in real time.
Masimo founder and CEO Joe Kiani said: “Since ORi’s availability and success outside the US, perioperative clinicians in the US have been waiting for a way to noninvasively monitor patients under supplemental oxygen beyond the limits of SpO2.
“We are thrilled that US clinicians can now integrate ORi monitoring, available now on our rainbow SET platform, into their oxygenation monitoring practices, alongside Masimo SET Measure through Motion and Low Perfusion pulse oximetry and experience their combined benefits.”
The clinical utility of ORi has been shown in multiple studies. In a study of 106 patients undergoing scheduled surgery, researchers observed a reduction in ORi to provide an advance indication of falling PaO2 when SpO2 is still greater than 98%.
In another trial, the researchers found that the use of ORi monitoring to titrate oxygen rates enabled a decrease in time spent with hyperoxia compared with the use of SpO2 alone.
With the De Novo, the medical technology company is expanding the RD family of pulse CO-oximetry sensors by releasing a new sensor line, the RD rainbow 4λ sensors.
