Radius PCG comes with Bluetooth connectivity for easy integration with Root platform to offer tetherless mainstream capnography
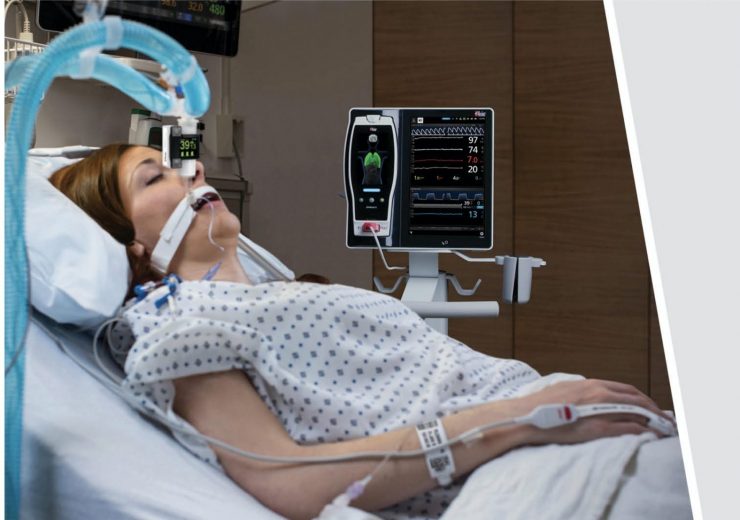
Masimo Root with Radius PCG. (Credit: Business Wire.)
Masimo has received the US Food and Drug Administration (FDA) 510(k) approval for its Radius PCG, a portable real-time capnograph with wireless Bluetooth connectivity.
Radius PCG wirelessly connects with the Root patient monitoring and connectivity platform to offer an easy tetherless mainstream capnography for patients of all ages.
Masimo’s Root is an expandable hub that integrates different technologies, devices, and systems to provide multimodal monitoring and connectivity solutions.
Root enables clinicians to simultaneously monitor the patients using Radius PCG and other measurements including Masimo SET, rainbow Pulse CO-Oximetry, O3 regional oximetry, and SedLine brain function monitoring.
Masimo founder and CEO Joe Kiani said: “With its wireless connectivity, Radius PCG is a powerful and useful tool for assessing end-tidal CO2 in a multitude of clinical scenarios.
“Masimo continues to make clinically relevant, accurate patient data available, helping clinicians gain the insights they need to make the best decisions and improve patient outcomes.”
Masimo said that its Radius PCG does not require routine calibration, and offers precise end-tidal carbon dioxide (EtCO2) and respiration rate measurements.
Also, the tool facilitates continuous display of EtCO2 waveforms within 15 seconds, all in a small, portable package that can fit in the palm of a hand.
Radius PCG, wirelessly connected to Root, offers cable-free capnography, automated documentation, and maximised data visibility and manipulation.
Temple University Hospital Philadelphia nursing associate vice president Joseph DiMartino said: “Radius PCG has been a game changer for our clinical team.
“It provides us with a portable and rapid measure of capnography for confirming airway placement in accordance with AHA guidelines.”
The other tetherless technologies offered by Masimo include Radius PPG, which offers Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, and Radius Tº that provides continuous temperature measurements.
In March, the company secured CE mark approval for a rugged handheld device called Rad-G with Temperature.
