MINIject is said to be a minimally-invasive glaucoma surgery device that is currently the only commercially available MIGS implant that targets the supraciliary space
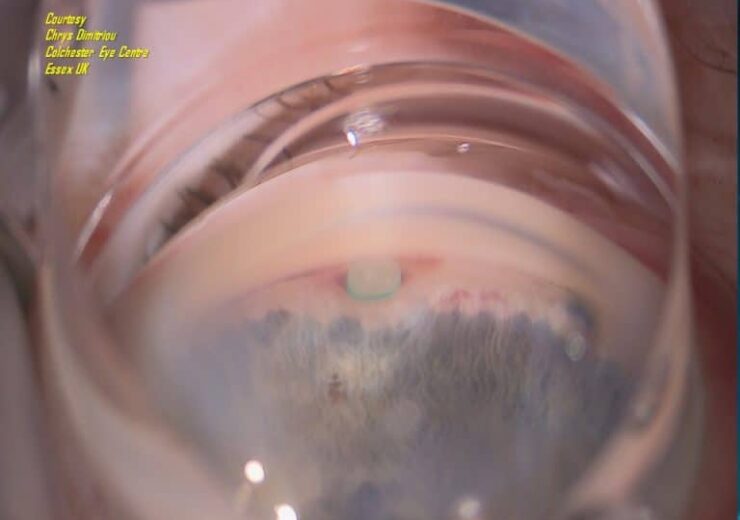
View of MINIject implanted in the supraciliary space. (Credit: iSTAR Medical/ Mr Chrys Dimitriou, Colchester Eye Center, Essex, UK)
ISTAR Medical has implanted the first patients in the STAR-VI international trial to evaluate the safety and efficacy of the MINIject implant in conjunction with cataract surgery of patients having primary open-angle glaucoma.
MINIject is said to be a minimally-invasive glaucoma surgery (MIGS) device that is currently the only commercially available MIGS implant that targets the supraciliary space.
The Belgium-based medical technology company will implant a total of 30 patients at up to seven sites in Europe and Central America in the prospective, interventional, multi-centre, single-arm study.
Under the STAR-VI trial, patients will be followed for two years after their cataract operation and concurrent implantation with MINIject.
According to iSTAR Medical, the primary endpoint of the trial is to calculate the proportion of patients achieving a reduction of ≥20% in diurnal intraocular pressure (IOP) from baseline to six-month follow-up, irrespective of the use of IOP-reducing therapies.
iSTAR Medical CEO Michel Vanbrabant said: “The initiation of our global STAR-VI trial is an important step to bring MINIject to more patients around the world, including to those with co-existing ocular conditions.
“We are committed to delivering our breakthrough eye care solutions to patients globally and look forward to reporting clinical data from this study in due course.”
MINIject combines the porous structure of its patented STAR material with the power provided by the supraciliary space.
The medtech company has designed the glaucoma therapy device to increase the natural fluid outflow and reduce the IOP and the need for medications.
Additionally, the device bio-integrates with the surrounding tissue to limit inflammation, fibrosis, and subsequent complications.
Furthermore, various clinical data have shown positive, consistent results up to two-year follow-up in patients across Europe, Asia, and Central and South America, iSTAR Medical claimed.
The MIGS device has already secured approval in Europe for the treatment of open-angle glaucoma and is seeking market approval in the US.
