The cloud-based, software-only medical device uses deep learning to spot and highlight findings associated with Aortic Atherosclerosis and Aortic Ectasia
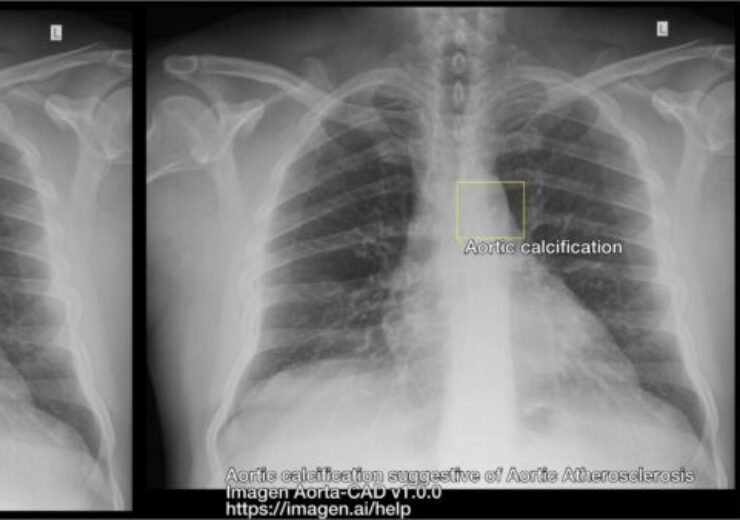
The output of FDA-cleared Aorta-CAD software. (Credit: PR Newswire/Imagen Technologies)
Imagen Technologies, an American medical technology firm, has received the US Food and Drug Administration 510(k) clearance for Aorta-CAD, its computer-assisted detection (CADe) device.
The cloud-based software-only medical device is designed to aid physicians in detecting findings on chest X-rays that indicate Aortic Ectasia and Aortic Atherosclerosis. For this, Aorta-CAD is said to use deep learning.
According to Imagen Technologies, the device is part of its Diagnostics as a Service (DaaS) platform. It is intended to help any chest X-ray-reading physician, including radiologists and primary care physicians (PCPs).
Aorta-CAD marks the fourth FDA clearance for the medical technology firm.
Imagen Technologies expects the addition of the newly approved device to its DaaS platform to help in earlier and better detection and treatment of chronic diseases.
Additionally, the Aorta-CAD device could help to identify previously undiagnosed chronic ailments, enhance the quality of life for patients, and lower overall healthcare costs.
Imagen Technologies CEO Alex Dresner said: “Imagen is on a mission to help Primary Care Physicians deliver faster, better diagnoses and care plans.
“By expanding our diagnostics as a service platform with Aorta-CAD, we expect to help PCPs significantly improve the quality of care and clinical outcomes for their patients with undiagnosed Aortic Atherosclerosis and Aortic Ectasia.”
Imagen Technologies said that the device after identifying findings on the chest X-ray image, generates annotations to bring attention to them through an overlay, which can be toggled off when not in use.
Aorta-CAD is said to integrate smoothly into current X-ray reading workflows without the requirement for costly physician retraining or new picture archiving and communication system (PACS) technology.
In its clinical trial, the software-only medical device delivered a 62% relative reduction by physicians in misses for aortic calcification suggestive of Aortic Atherosclerosis. It also showed a 35% relative reduction in misses for dilated aorta suggestive of Aortic Ectasia.
When assisted by the device, 96% of physicians showed improvement, said Imagen Technologies.
