The IB Lab LAMA software combines deep learning technology and advanced software engineering to provide rapid, accurate and standardised radiological modular musculoskeletal (MSK) parameters on X-rays
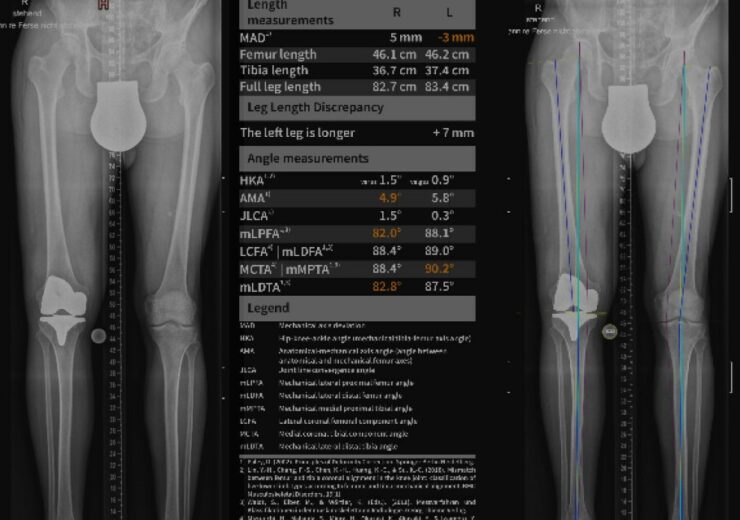
IB Lab LAMA Sample Outputs. (Credit: PRNewswire/ImageBiopsy Lab)
Austria-based medical software company ImageBiopsy Lab (IBL) has secured the US Food and Drug Administration (FDA) approval for its IB Lab LAMA image processing software.
IB Lab LAMA is a fully automated radiological image processing software intended to measure the geometric length and angles of the lower limb on full-leg X-rays.
According to the company, accurate and standardised measurements to assess the lower leg geometry are essential before and after surgery.
However, surgeons are often burdened due to large caseloads and busy practices.
IBL said that its software expedites image-based workflows of surgeons and radiologists alike, by combining deep learning technology and advanced software engineering.
It provides rapid, accurate and standardised radiological modular musculoskeletal (MSK) parameters on X-rays, compared to fixed predetermined norm ranges.
The IB Lab LAMA software summarises the outputs in detailed reports that can be viewed on any regulatory-approved medical DICOM viewer.
IB Lab co-founder and CEO Richard Ljuhar said: “FDA clearance serves as a significant validation of the accuracy and quality of our LAMA module.
“It is a huge milestone to bring AI-supported software tools to surgeons, not only to increase efficiency, but also to improve the outcomes and follow-ups for their patients.”
ImageBiopsy Lab is engaged in developing AI-driven solutions that provide radiologists and orthopaedics with rapid, quantitative, and standardised reports of relevant measurements.
The company’s portfolio of products includes solutions for knee, hip, hand, leg, and spine.
In addition, ImageBiopsy Lab is currently providing solutions for fracture detection, further enhancing its comprehensive range of solutions.
The company previously secured FDA approval for its IB Lab KOALA Knee-Osteoarthritis Labelling Assistant, with plans to further expand its MSK-focused software solutions to the US.
Standalone Performance Study principal investigator Avneesh Chhabra said: “Artificial intelligence’s potential role in orthopaedic surgery is significant.
“Using deep learning, we aim to support pre- & post-operative decision management, representing a potential key for a more personalised treatment pathway for each patient.”
