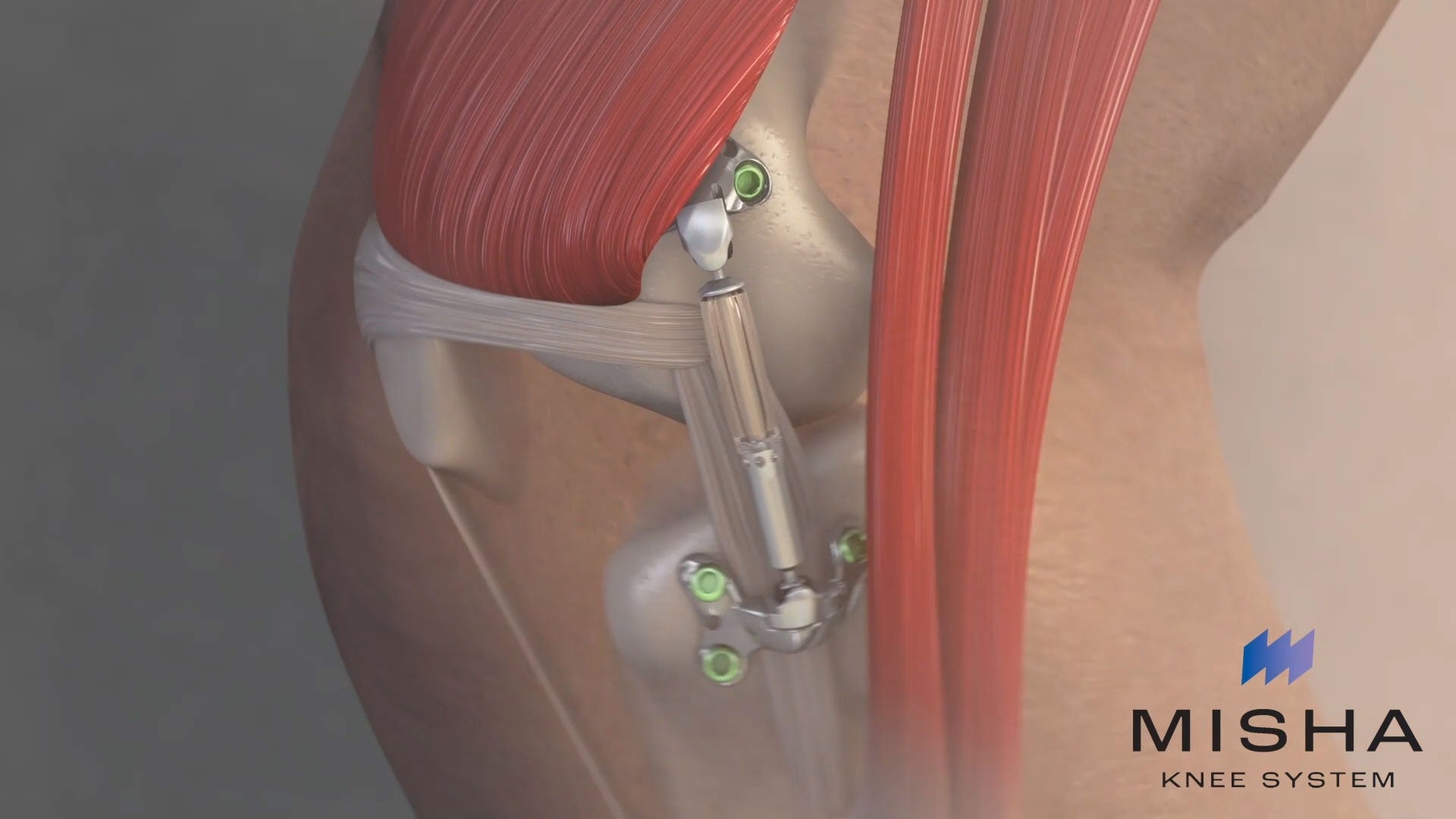
Moximed, a medical device company focused on knee osteoarthritis (OA), has received the US Food and Drug Administration (FDA) marketing authorisation for its MISHA Knee System.
MISHA is an implantable shock absorber (ISA) for the knee, implanted during an outpatient-compatible procedure, for the treatment of medial compartment knee OA.
It is indicated for people with medial knee OA, for whom non-surgical or surgical treatment is ineffective, and are ineligible for joint replacement due to age or disease progression.
Moximed said that the MISHA Knee System has been designed using clinically established benefits of load reduction on diseased joints.
In a recent clinical study, the system showed superiority over high tibial osteotomy (HTO), a well-established surgery with decades of proven clinical results, said the company.
Moximed founder and CEO Anton Clifford said: “This is a milestone event for knee OA sufferers, and it’s the result of unwavering clinical research and development that spans more than 10 years.
“Also, we recognise the dedicated reviewers at FDA for completing their thorough benefit-risk assessment of our breakthrough technology. We’re thrilled to now be in a position to make the surgery available to patients.
“We are committed to providing excellent medical education and customer service, supporting the selection and treatment of indicated patients, and demonstrating the scalability of our business as we introduce the MISHA Knee System to the US.”
Moximed said that its MISHA Knee System is the first implantable shock absorber to reduce weight on the knee joint with every walking step.
It is a result of the company’s more than a decade of clinical research and development.
Placed on the medial knee, the implant moves with the natural joint, reducing about 30% of the peak force on the knee with every walking step, said the medical device company.



